Clinical Use of Flow Cytometry in Pediatric Cancer Diagnosis Webinar
Today I will share our experiences at Moi Teaching and Referral Hospital (MTRH) to give an overview of the problems in the cancer diagnosis. We will focus on the delays and lack of technology to make a diagnosis of cancer, especially acute leukemia and lymphomas and look at whether flow cytometry can be a solution for diagnosis.
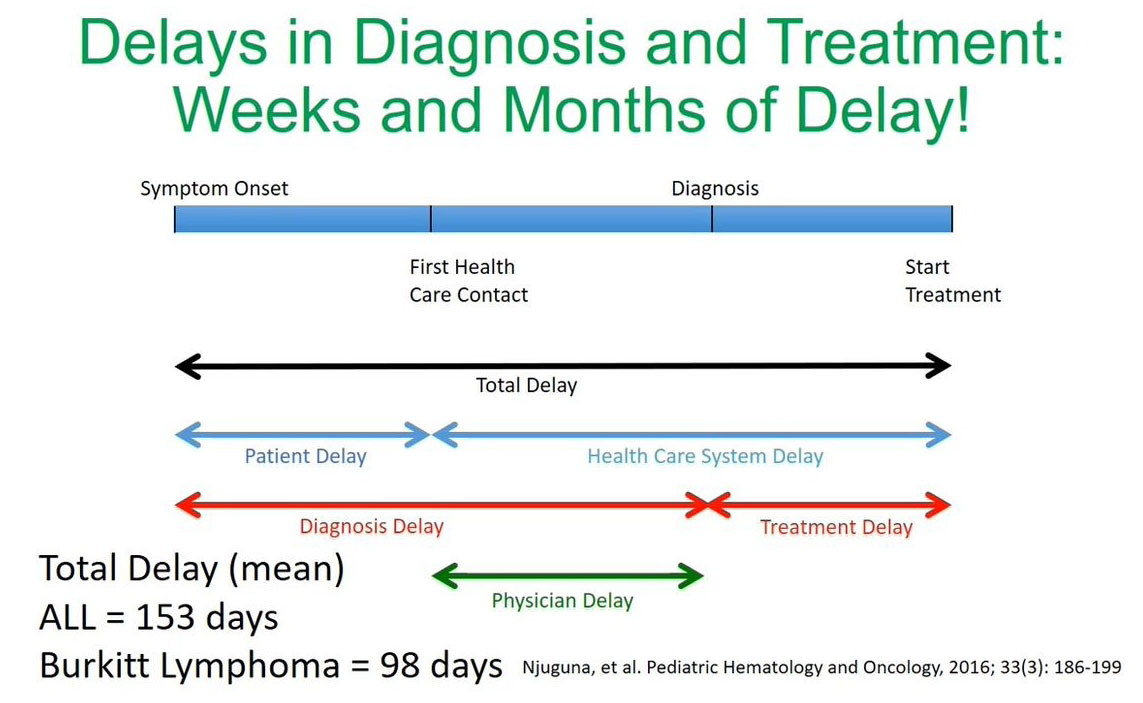
Delays in Diagnosis & Treatment
From our experience and the data published by our colleague Dr. Njuguna et al. we've noticed that the diagnosis challenges are due to the delays making a diagnosis and starting or initiating treatment.
In this study published recently by Dr. Njuguna et al. the total delay time was significant. If you look at acute lymphoblastic leukemia (ALL) we counted 153 days from making the initial diagnosis at symptom onset to starting treatment and for Burkitt’s lymphoma, it was found to be 98 days. What is significant is that a lot of these delays are in the health care system either when making a diagnosis or between diagnosis and the initiation of treatment.
Cancer Challenges in High Income Countries
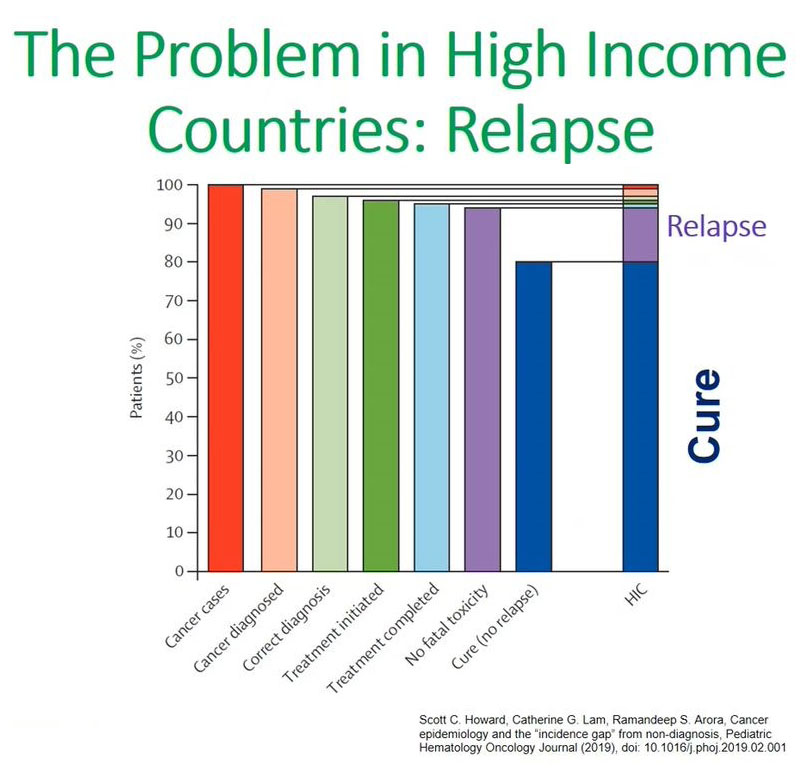
This made me think about this graph that was presented by a colleague, Scott Howard from Saint Jude's, and refers to the issues of cancer in children in high-income countries.
From these bar graphs what we can appreciate is that in high-income countries the main issue is relapse. High-income countries can cure 80 to 85% of children diagnosed with cancer and their main concern is relapsing patients, and that is the 15% that most studies are focusing on.
Cancer treatment failure low-middle income countries
This contrasts significantly with what we see in low-income countries, as we see in the next graph.
When you look at low-income countries, which includes MTRH where we are located, the main issue that inhibits curing patients is no diagnosis. When you make a diagnosis sometimes or most of the time these diagnoses are wrong in terms of the actual diagnosis. This is also coupled by issues of once a diagnosis has been made, a significant percentage of the patients will refuse or abandon treatment. All these contribute to making a cure less than 20% in children in the low- and middle-income countries.
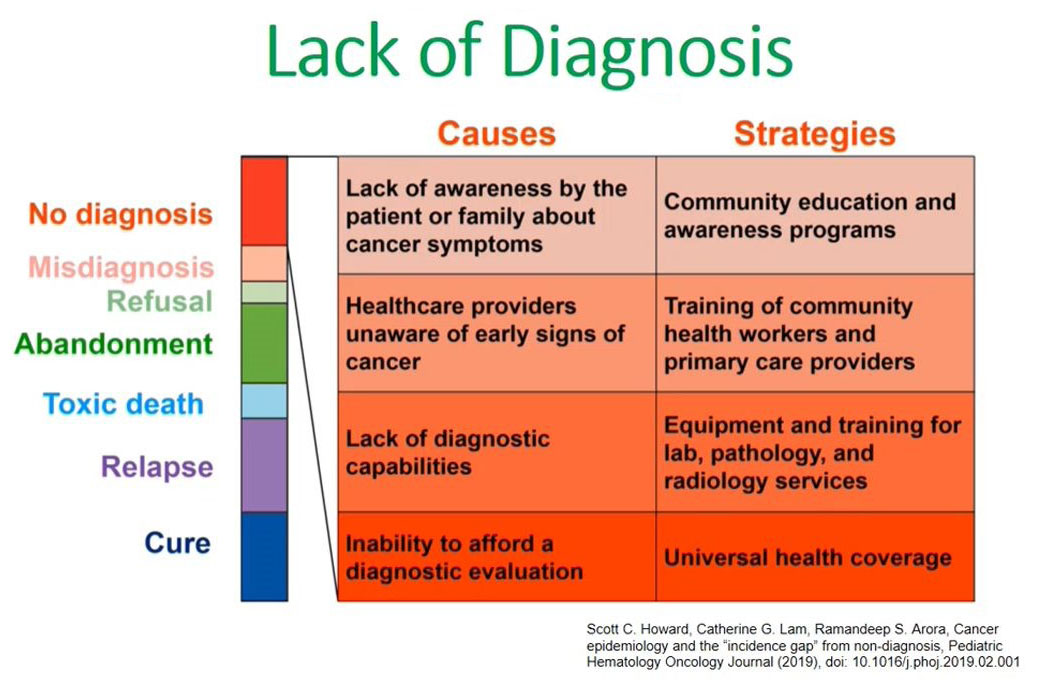
Lack of diagnosis
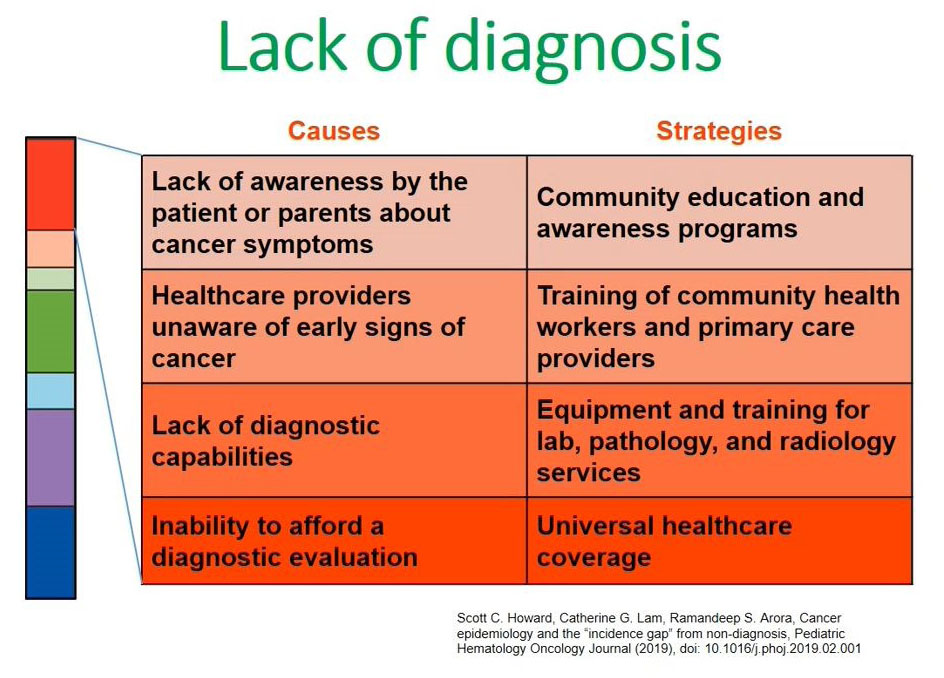
What are the causes of this lack of diagnosis in low and middle-income countries? Again, I refer to this publication made by Scott Howard.
The left side of the diagram shows the causes for a lack of diagnosis in our setups and the second point states health care providers being unaware of the early signs or symptoms of children presenting with cancers; the third point is the lack of diagnostic capabilities which includes lack of technological diagnostic capacities like flow cytometry and in most low- and middle-income countries this is coupled with the inability to pay for this diagnostic evaluation. There are less facilities available and the majority of our clients are unable to pay for them.
Leukemia as a Model for Difficulty in Diagnosis
Moi Teaching and Referral Hospital is my hospital in Western Kenya and is one of the institutions Steve Kussick alluded to. The population served by this facility is 20 to 25 million and we expect to see about 300 cases of acute lymphoblastic leukemia every year. However, in the years 2010 to 2016, we were only able to see 20 to 30 cases per year. This made us ask ourselves if we could pick up more cases from the community and if we are able to sort these issues of diagnosis?
Leukemia: Is it feasible to screen for early detection?
In our pilot study we looked to see if it was feasible to screen for leukemia in the community. We know that in high income countries a full hemogram and peripheral blood smear is the first step to making a diagnosis of acute leukemia. However, in our setups, especially in Western Kenya there more malaria slides prepared compared to a peripheral blood smear.
We asked ourselves whether it would be possible to pick these cases of leukemia out there in the community by looking at a malarial smear which is 10 times more commonly prepared than a full hemogram.
We proceeded to do a retrospective malarial slide review and close to 32,000 slides were reviewed looking for evidence of higher white blood cell counts or presence of neutropenia that's less than 20% neutrophils. Once this was recognized, the high-power confirmation was done, and a photograph taken for reference or for looking at the WBC differential which we were able to do in 549 cases out of 32,000 slides that were reviewed initially. Then we proceeded to isolate the DNA to look for clonality either B cell or T cell clonality for 194 cases.
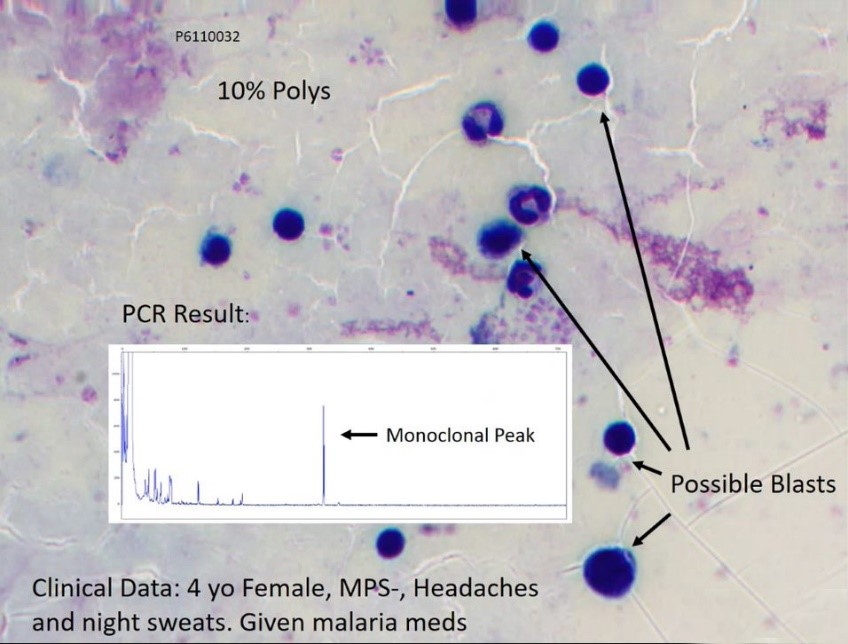
What we found when looking at the PCR results for this case was evidence of monoclonal or clonality. As you can see here there was monoclonal peak which was consistent with a B or T cell clonality. This is an example morphology of a four-year-old female patient who had 10% polys and when we looked at the clinical history she presented with headaches and night sweats. When they looked at the malarial parasite it was negative, so the patient was given malarial medication and discharged but was not followed up to look for the possible presence of blasts, as we see in the morphological picture.
In this pilot study we were able to review 44 slides that had signs or features of clonality or clonal PCR products being detected. Out of this, we confirmed that using this method it was possible to screen acute leukemia in the community and we were able to elucidate that it was possible to pick these extra cases that were not coming to the health care facility.
Flow cytometry at Moi Teaching & referral Hospital
This has been well described by Steve Kussick in the previous slide. This is a color plot not from one of the patients that we have presented but from a patient that had leukemia. This flow cytometry we can do at MTRH at a cost of $140 U.S. dollars, which if you compare, is almost the same price as a CAT scan or CT scan in the same facility.
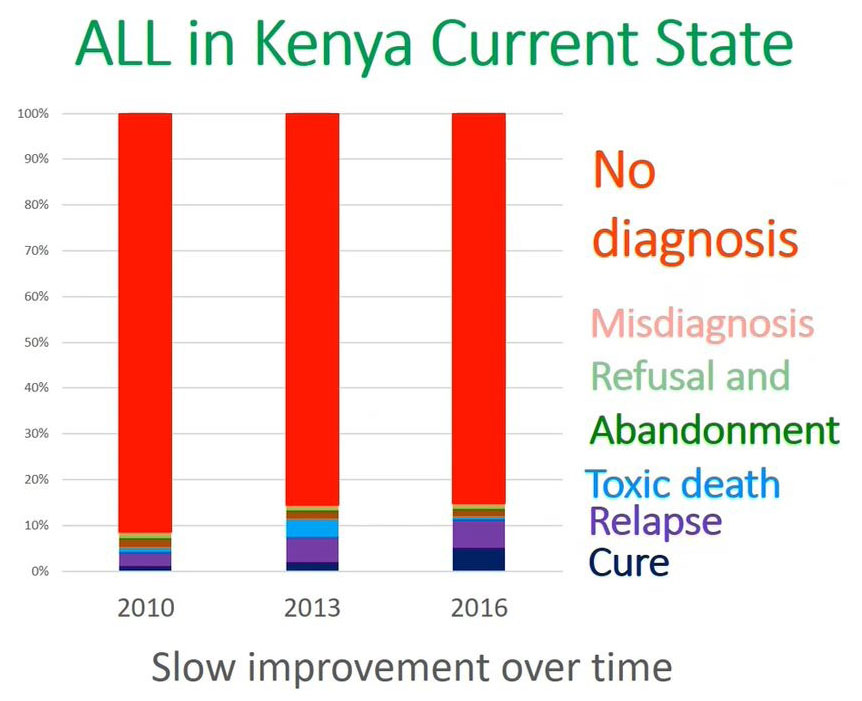
ALL Diagnosis in Kenya
What is the current state of acute lymphoblastic leukemia in terms of diagnosis in Kenya?
This is a representation from the 20 to 25 million population in Western Kenya. At MTRH as we have alluded from 2010 to 2016 the most important issue is no diagnosis and cure is the lower blue which is less than 10% of the patients were able to cure.
The reason why we are not able to achieve a cure in Kenya or in our setup is because you are not able to diagnose a significant population of patients in the community or for those patients who come to the facility.
Returning to this graph we ask what strategies can we use to address this issue of a lack of a diagnosis which is contributing to poor outcome in our low- and middle-income facility?
We have addressed the causes of a lack of diagnosis and one strategy to address the issue of health care providers not knowing the signs of cancer is to train them at the community level.
Other issues are the lack of diagnostic capabilities, and this we can address by equipping and training lab pathology and radiology services in these new diagnostic facilities like flow cytometry. The third aspect is universal health coverage, which is being advocated for by several organizations, including governmental organizations.
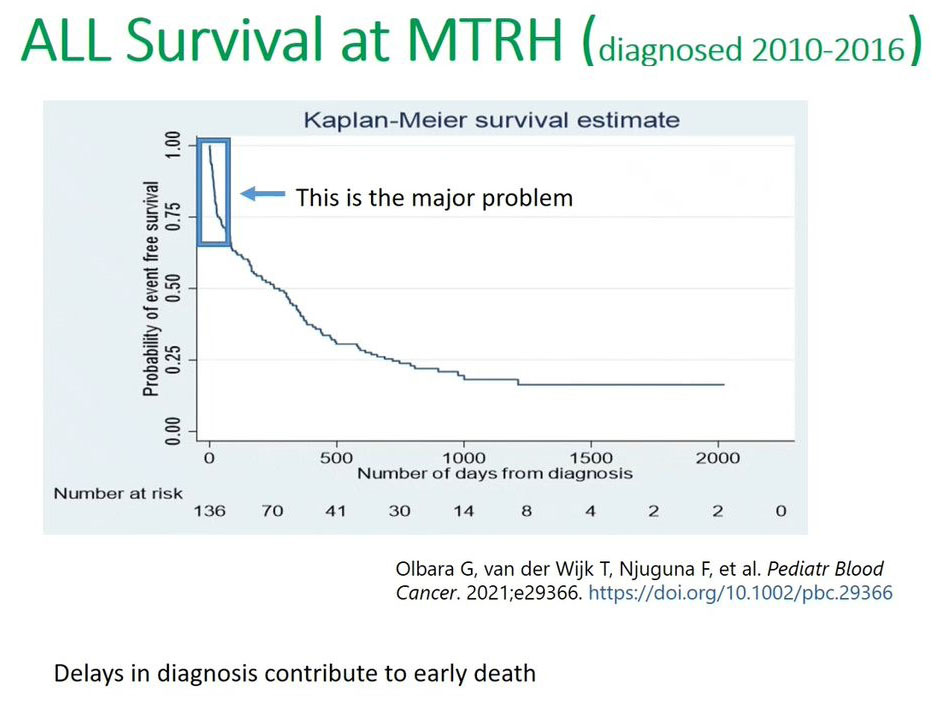
This recent publication in the 2021 Pediatric Blood Cancer (PBC) journal, and we can see the Kaplan-Meier curve showing very low survival, less than 25% survival for those children diagnosed between 2010 and 2016. But the key points that I want to bring forth is this steep decline in terms of the Kaplan-Meier curve in the first months or the first 100 days. This is where the major problem occurs because of this delayed diagnosis or misdiagnosis we lose a lot of patients. It's postulated that a lot of patients are lost or do not survive the first 100 days after their diagnosis and from the time when they enrolled in the hospital.
In conclusion, what we have learned when we look at leukemia is that flow cytometry can be very useful in terms of speeding up diagnosis and approaches with the community so that they send a blood sample, or the bone marrow are sent for diagnosis instead of sending the patient. After their diagnosis has been made by flow cytometry then they can refer this patient.
Then the second aspect is to address issue of continued education in the community, to help them identify the signs and symptoms of leukemia and then build up a referral network to help reduce the delays and improve the survival outcome. We are trying to do this using the project ECHO platform to link up the community workers or the primary and secondary level health care providers.
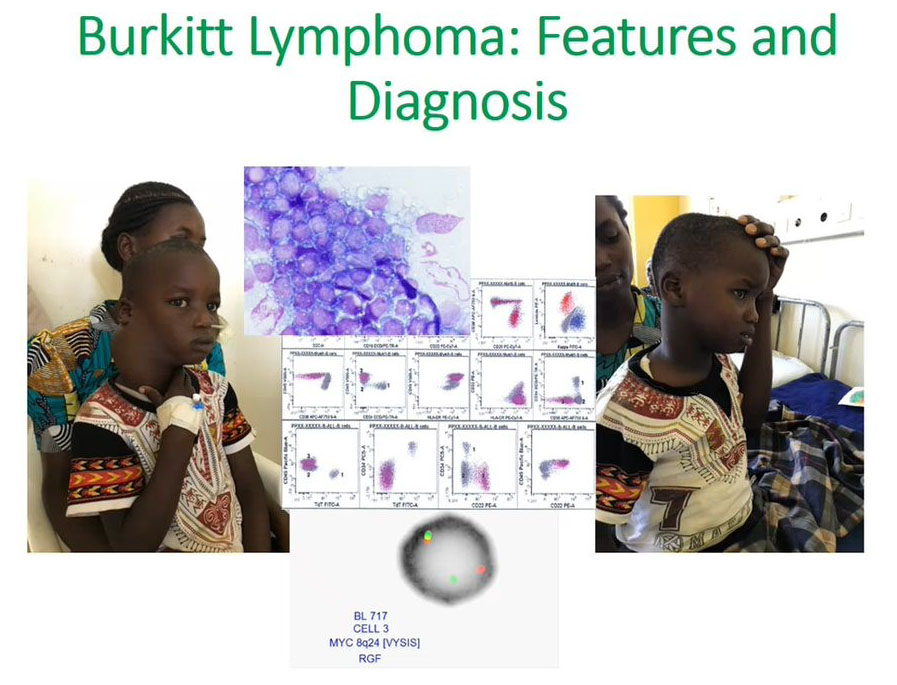
Burkitt’s Lymphoma features and diagnosis
The second aim was to understand how flow cytometry can be useful for looking at Burkitt’s lymphoma.
We appreciate that it will present as a mass as we see on the left side of the face and it would be easy to get tissue and do flow cytometry for diagnosis. Once we make this diagnosis, we can initiate treatment early. It is possible to use flow cytometry from this tissue to make a diagnosis. The right photograph shows a successful patient outcome after initiation of treatment.
1. The first one is to look at how we can improve diagnosis and staging or Burkitt’s lymphoma:
a. Improve speed and accuracy with flow cytometry and FISH
b. Reduce to diagnosis and permit initiation of therapy in one week or less
c. Earlier initiation of therapy to lead to improved clinical outcomes
2. The second aim was to address the issue of abandonment
a. Weekly phone contact with families
b. Payment for transportation to the hospital for planned chemotherapy
c. Assist with loss of income by providing #$1/day during the six-month course of therapy.
Study results
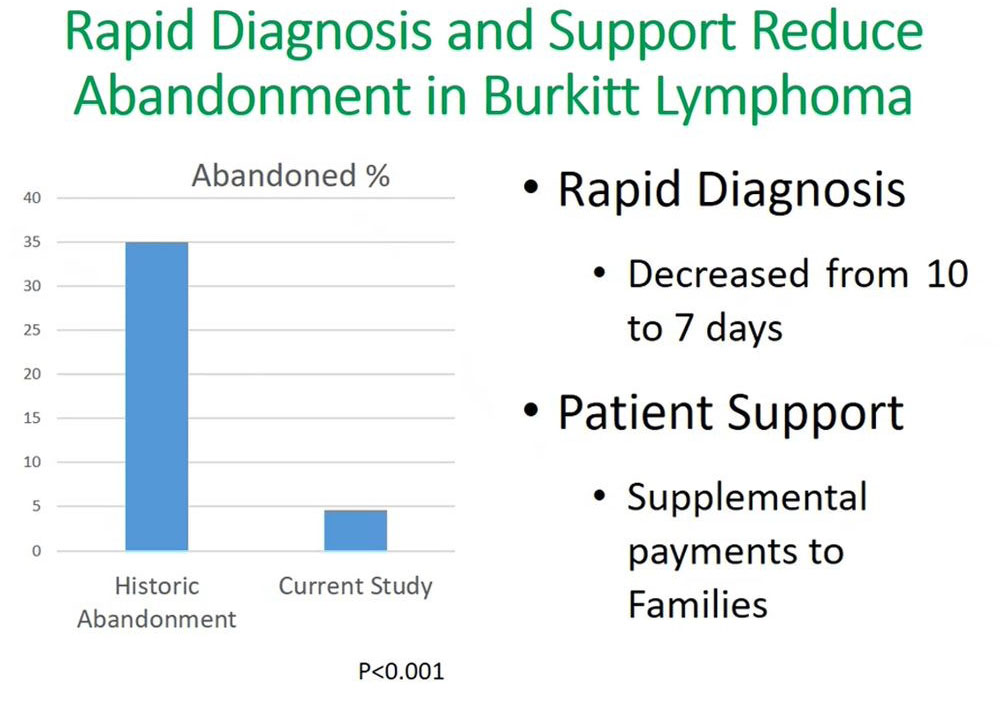
In this sample study we recruited 94 patients in the first portion of the study, the diagnostic portion and out of this 43 of the patients had Burkitt’s lymphoma. Then 43 were enrolled in the second part where they were financially supported through their course of treatment.
Our findings from this study showed that by supporting the patient through the six months of treatment at our facility, there was significant decline in abandonment, reducing from 35% to less than 5%. An important outcome of this was that the time to diagnosis decreased from 10 days to seven days. This rapid diagnosis used flow cytometry, which reduced the turnaround time equally for receiving results and initiating treatment for patients.
Survival improvement in Burkitt’s Lymphoma
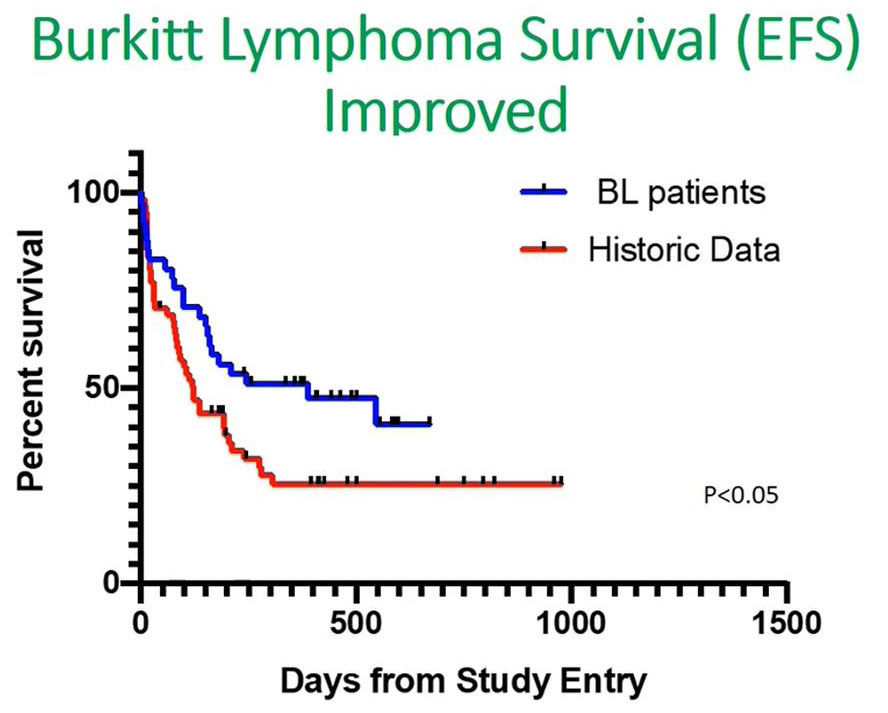
When we looked at our data, you can see from the bluelines there was a significant increase in event free survival compared with an historical cohort shown in red.
Looking at the first 100 days, there was a sharp decline, and this made us believe from this data that if we address the initial issues of delays in our patients, even in Burkitt’s lymphoma, then we'll be able to improve the overall survival outcome.
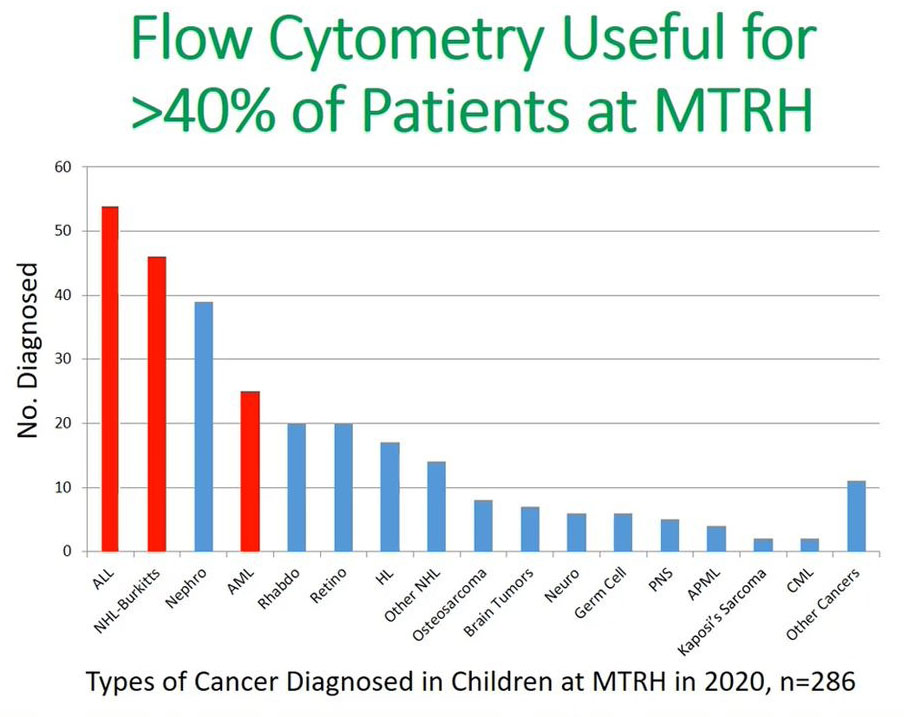
This is the most current data, 2020 data for all children diagnosed with cancer at MTRH and we can see by improving our diagnosis and our communication with the health care providers in the community we were able to increase the numbers of acute leukemia that were being diagnosed by 20–30 to 54 and this applied the same to their Burkitt’s lymphoma.
It is paramount to say that flow cytometry can be useful in assisting in improving diagnosis or reducing the delays in diagnosis in more than 40% of the patients in MTRH. This is represented by the three cohorts: acute leukemia, non-Hodgkin’s and acute myeloid leukemia (AML) patients and in these patients will be able to make a diagnosis using flow cytometry. Flow cytometry is useful in the diagnosis of leukemia and lymphoma in Kenya, and that the fact that technology is improving as has been alluded in the previous presentation.
We also need to continue training the Regional Health Care providers to recognize the potential patients with leukemia and lymphoma. This can be achieved through channels like Project Echo in the community or continued medical education on the early signs of cancer. We also need to develop systems for obtaining samples of blood, bone marrow and fine needle aspirates of masses for Burkitt’s lymphoma and be able to transport them to a central lab to help improve the rapidity of diagnosis.
All said and done, costs remain a barrier, but this can be supported by the universal healthcare provision or health insurance schemes.
In the last slide I want to acknowledge the ten years of collaboration between Moi Teaching and Referral and Moi University and the colleagues in my facility. Then also Indiana University, led by Professor Terry Vik, who provided a lot of input. Then other collaborators, including Princess Maxima Hospital, Netherlands. UMass Medical Center, JOOTRH and Ampath, and finally Burkitt’s Lymphoma Fund who have supported our pathology team in improving the technology of flow cytometry.