Panel Design and Marker Combinations of ClearLLab 10C
The first FDA cleared 10-color leukemia & lymphoma* immunophenotyping panel
Sandra Hernandez, Global Product Manager, Beckman Coulter Life Sciences
Clinical cytometry challenges
There are many challenges in the clinical flow cytometry laboratory. Regulatory requirements are increasingly being addressed by the compliance requirements from different agencies, and this is an issue because there are no in vitro diagnostic (IVD) assays other than ClearLLab. There is a need to streamline workflows; unfortunately, however, assay validations, reagent quality control (QC) and cocktail preparations are all tedious manual processes that require expertise and resources.
There is a two-pronged drive to improve efficiency because manual pipetting can lead to potential errors, and assay QC cocktails require extensive inventory control management.
With more colors comes increasing complexity, and in clinical flow cytometry we are moving forward into these multicolor applications. Standardization is also an ongoing challenge because there are many non-standardized lab-developed test (LDT) assays, and these assays require special trainings and resources plus valuable time to train laboratory staff.
Leukemia & Lymphoma Cases
With increasing complexity and multicolor applications comes the added challenge of data management, and it's important to be able to analyze qualitative data in the most efficient manner possible. Our goal was to develop a panel that addressed these clinical flow cytometry lab challenges and provide an efficient workflow for identifying normal versus abnormal phenotypes.
In the US and EU, the flow cytometry cases that come into the leukemia and lymphoma laboratory can be segregated, and, out of the total cases, we expect that about 50% will come with a normal phenotype and the other 50% will be an abnormal phenotype. Of the abnormal phenotypes, 70% will be lymphoid and 30% myeloid. Of the 70% that are lymphoid, 85% will be B cell lineages and the remaining 15% will be other lineages.
Our solution is not only a solution for our panels, it's also a system approach.
Starting with the ClearLLab 10C panels, and I really want to go over this because I'm not sure if everybody is familiar with the complete 10-color system, so I will briefly go through the different components of the system. These are 10-color, four-antibody panels in dry unitized premixed tubes. These tubes come in 25 tests per kit and are lineage-specific; there’s a B cell tube, a T cell tube and two myeloid tubes, M1 and M2 respectively.
What's unique to the system is that it has control cells. These are true process controls and are processed in the same manner as your samples. There are two phenotypes, normal and abnormal. In addition, there is a compensation set up kit, also in the same dry format, which has five setups. To enhance this compensation setup, there are compensation beads, which are antibody capture beads. There are also sample prep reagents, which include the IOTest3 Lyse reagent, with 100 tests per kit, and the IOTest fixative. Again, as part of this enhancement of the compensation, there is advanced compensation setup software for both Navios and Navios EX instruments. Bringing this all together is the Kaluza C analysis software, which helps to streamline the analysis process with downloadable templates to help facilitate analysis. Kaluza C is a clinical IVD analysis software.
The ClearLLab 10-color panel is intended for in vitro diagnostic use, and for qualitative identification of cell populations by multiparameter immunophenotyping on Navios and Navios EX cytometers. These reagents are used as an aid in the differential diagnosis of hematologically abnormal patients who have, or are suspected of having, these haemopoietic neoplasms: chronic leukemia, acute leukemia, non-Hodgkin’s lymphoma, myeloma, myelodysplastic syndrome or myeloproliferative neoplasms.
These reagents can be used with peripheral whole blood or bone marrow collected in EDTA, ACD and heparin, so you have a wide choice of the major anticoagulants available, and lymph node specimens. As with all flow cytometry data, this should be interpreted by a pathologist or equivalent professional, in conjunction with other clinical and laboratory findings.
1. Know your cytometer
2. Define panel
3. Select antibodies and fluorochromes
4. Optimize antibody selection
5. Minimize spectral overlap with compatible fluorochromes
6. Use appropriate controls
7. Well-designed panels and quality reagents are a must!
Tips for panel design
I know that designing these panels is not easy, so here I'd like to provide some tips on panel design.
First, you need to know your cytometer, because you know you need to develop your panels based on your cytometer. In this case, panels were designed for Navios and Navios EX cytometers.
You need to define this panel, and this can be a time-consuming process because you need to look at literature and guidelines, and then you need to determine what type of panels you want to incorporate into your testing.
You need to select the appropriate antibodies and fluorochromes, and this is important because it's critical to match the brightness and the antigen density. If your dye is too dim, it leads to poor sensitivity, so you need better sensitivity for weak antigens. If dyes are too bright, it leads to strong spillover, so you want less spillover from these strong antigens. Well-designed panels and quality reagents are foundational in order to have a successful panel.
With the ClearLLab 10-color panel, all this work has been done for you, and ensures fully validated FDA‑cleared panels.
ClearLLab 10C Panel Configurations
For ClearLLab panel configurations, I want to explain the panel in its entirety first and tell you a little bit more about how this configuration came about.
The specific choices and combinations in ClearLLab 10-color panels are based on the guiding principles of, first, addressing clinical indications as we saw in our intended use, and second, we should be accounting for all major cell populations in the sample. Finally, we need to provide sufficient comprehensive identification of all major categories of hematopoietic cell populations in both normal and neoplastic states.
Table 1. ClearLLab 10C Panel Configurations. Both mature and immature markers are combined in the panels to provide efficiency as well as allowing for the identification of all cells in the tubes. The construction of the panel is centered on developing individual tubes within lineages that can be combined to yield an informative immunophenotype.
| Blue Laser | Red Laser | Violet Laser | ||||||||
| FITC | PE | ECD | PC5.5 | PC7 | APC | APC-A700 | APC-A750 | PB | KrO | |
| B Cell Tube | Kappa | Lambda | CD10 | CD5 | CD200 | CD34 | CD38 | CD20 | CD19 | CD45 |
| T Cell Tube | TCRgd | CD4 | CD2 | CD56 | CD5 | CD34 | CD7 | CD8 | CD3 | CD45 |
| M1 Cell Tube | CD16 | CD7 | CD10 | CD13 | CD64 | CD34 | CD14 | HLA-DR | CD11b | CD45 |
| M2 Cell Tube | CD15 | CD123 | CD117 | CD13 | CD33 | CD34 | CD38 | HLA-DR | CD19 | CD45 |
Looking at the panel configuration, one of the things we have done is built in a little bit of redundancy, which is useful because it gives labs an added QC with the panel. ClearLLab has CD45 in all of the four tubes, which is important as a gating marker. It has CD34 in all four tubes, which is useful as an indication of an immature population. The panels not only provide markers for mature, but also immature blast-like populations that might be present in the sample. There is some redundancy in the myeloid tube, such as granulocyte monocyte marker CD-13, and in the monocyte marker HLA-DR, which is important in some of the different acute myeloid leukemias (AMLs), especially for AML M3 or acute promyelocytic leukemia (APL). There is also some redundancy with CD19, which is appears in the B cell tube and in the M2 tube (which I will explain later) as well as CD7, a T cell marker, incorporated in the M1 tube, and I will also talk about that later.
The important point is that both mature and immature markers are combined in ClearLLab panels to provide efficiency, as well as enabling identification of all cells in the tubes. That means normal and abnormal cells that might be present. The construction of the panel is centered on developing individual tubes within the lineages, and we have covered the major lineages, B, T and myeloid that can be combined to yield an informative immunophenotype.
ClearLLab 10C Panel Design
The panel was designed to include primary antibodies that are lineages oriented in the 2006 Bethesda Consensus, which labs in the United States are familiar with. The combinations are intended to identify leukocyte sub-populations that express these antigens alone or in a specific co-expression pattern.
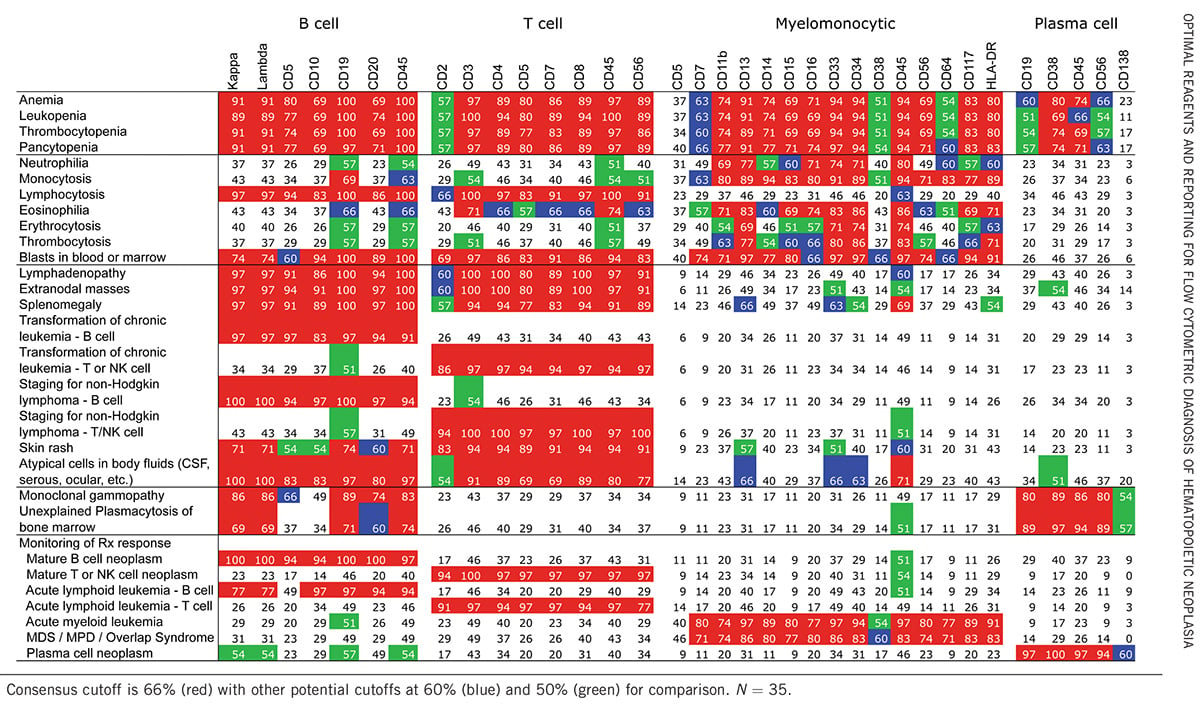
Table 2. Results of Consensus Survey of Reagents Useful for Initial Evaluation for Hematopoietic Neoplasia.2
Table 3. Consensus Reagents for Initial Evaluation for Hematopoietic Neoplasia.2
| Lineage | Primary Reagents |
| B cells | CD5, CD10, CD19, CD20, CD45, Kappa, Lambda |
| T cells and NK cells | CD2, CD3, CD4, CD5, CD7, CD8, CD45, CD56 |
| Myelomonocytic cells | CD7, CD11b, CD13, CD14, CD15, CD16, CD33, CD34, CD45, CD56, CD117, HLA-DR |
| Myelomonocytic cells (limited) | CD13, CD33, CD34, CD45 |
| Plasma cells | CD19, CD38, CD45, CD56 |
2006 Bethesda Consensus markers
Now we are going to compare the ClearLLab 10-color system versus the Bethesda Consensus, which breaks down their primary reagents by the lineages. In the B-cell, the primary markers are CD5, CD10, CD19, CD20, CD45, Kappa and Lambda. If you look at the ClearLLab B cell tube, all those markers are included as primary markers, in addition to other markers that we've decided to incorporate. I'll talk a little bit about that when I talk about the individual tubes.
For T cell and NK-markers, we see CD2, CD3, CD4, CD5, CD7, CD8, CD45 and CD56, and these are also in the ClearLLab 10-color T cell tube. In addition to TCR Gamma-Delta, which is a secondary reagent, ClearLLab incorporates CD34 to enable identification of an immature blast-like population if it's present in the sample.
Table 4 and 5. ClearLLab 10C vs Bethesda Consensus.
| Blue Laser | Red Laser | Violet Laser | ||||||||
| FITC | PE | ECD | PC5.5 | PC7 | APC | APC-A700 | APC-A750 | PB | KrO | |
| B Cell Tube | Kappa | Lambda | CD10 | CD5 | CD200 | CD34 | CD38 | CD20 | CD19 | CD45 |
| T Cell Tube | TCRgd | CD4 | CD2 | CD56 | CD5 | CD34 | CD7 | CD8 | CD3 | CD45 |
| M1 Cell Tube | CD16 | CD7 | CD10 | CD13 | CD64 | CD34 | CD14 | HLA-DR | CD11b | CD45 |
| M2 Cell Tube | CD15 | CD123 | CD117 | CD13 | CD33 | CD34 | CD38 | HLA-DR | CD19 | CD45 |
| Lineage | Primary Reagents | |||||||||
| B cells | CD5, CD10, CD19, CD20, CD45, Kappa, Lambda | |||||||||
| T cells and NK cells | CD2, CD3, CD4, CD5, CD7, CD8, CD45, CD56 | |||||||||
| Myelomonocytic cells | CD7, CD11b, CD13, CD14, CD15, CD16, CD33, CD34, CD45, CD56, CD117, HLA-DR | |||||||||
| Myelomonocytic cells (limited) | CD13, CD33, CD34, CD45 | |||||||||
| Plasma cells | CD19, CD38, CD45, CD56 | |||||||||
Table 6. Reagents for Secondary Evaluation of Specific Hematopoetic Cell Lineages.
| Lineage | Secondary Reagents |
| B cells | CD9, CD11c, CD15, CD22, cCD22,CD23, CD25, CD13, CD33, CD34, CD38, CD43, CD58, cCD79a, CD79b, CD103, FMC7, Bcl-2, cKappa, cLambda, TdT, Zap-70, cIgM |
| T cells and NK cells | D1a, cCD3, CD10, CD16, CD25, CD26, CD30, CD34, CD45RA, CD45RO, CD57, αβ‑TCR, γδ‑TCR, cTIA-1, T-beta chain isoforms, TdT |
| Myelomonocytic cells | CD2, CD4, CD25, CD36, CD38, CD41, CD61, cCD61, CD64, CD71, cMPO, CD123, CD163, CD235a |
| Plasma cells | CD10, CD117, CD138, cKappa, cLambda |
For myelomonocytic cells, the ClearLLab 10-color system has CD7, CD11B, CD13, CD14, CD15, CD16, CD33, CD34, CD45, CD56, CD117 and HLA-DR, the primary reagents in the Bethesda Consensus, and these markers are all represented within the M1 and M2 tubes — in addition to some redundant and secondary markers, and CD123 and CD117 as secondary markers, which can also be used for plasma cell identification.
ClearLLab doesn’t have a specific plasma cell tube, but based on the primary reagents for plasma cells, which according to Bethesda Consensus are CD19, CD38, CD45 and CD56, it has those markers within the panel. Therefore, ClearLLab can identify these, and then a confirmatory or reflex panel is used to confirm if they are normal plasma cells or malignant myeloma cells.
This is a comprehensive panel; however, it is not the be-all and end-all panel. There will be instances where labs will need to run disease-specific reflex tubes to confirm what is seen with the ClearLLab panel. For example, in the case of hairy cell leukemia, because ClearLLab doesn’t have the prerequisite CD103 in the panel, labs would need to run a reflex. Still, this is a comprehensive panel that cuts down the total number of reflex panels you need to run because it provides a snapshot of the lineages, providing labs enough information to get them 80% to 90% of the way to a diagnosis of abnormal phenotypes with minimal reflexes required.
B cell markers
Now I'm going to look specifically at the individual tubes, at the markers, and the utility of having these marker combinations in the tubes.
Starting with the B cell tube, ClearLLab has CD10, which can be useful for precursor B cell leukemias and on a subpopulation of some mature markers. On the B cell lineage there is CD5, which is really a T cell marker, however this is also expressed in a subpopulation of B-cells. With CD5 and CD10, those markers are useful to differentiate the different B-lymphoproliferative disorders.
We have CD200, which is a B cell marker. One of the questions I’m most frequently asked when I talk about the panels is why do we have CD200 and not the traditional CD23? What we found during our research and development, when reviewing publications, was an increase in the use of CD200 and chronic lymphocytic leukemia (CLL).
Table 7. ClearLLab 10C System Specificity B Cell Tube: Leukocyte Typing International Workshop Information for the B Cell Tube Markers.
| Marker | Clone/Ig Chain (species) | Leukocyte Typing International Workshop | Reactivity |
| CD10 | ALB1 IgG1 (mouse) | Third | Common acute leukemia antigen (CALLA), lymphatic precursor cells, neutrophils, subpopulation of mature B Cells |
| CD5 | BL1a IgG2a (mouse) | Third | Thymocytes, mature T Cells, subpopulation of B Cells |
| CD200 | OX-104 IgG1 (mouse) | Seventh | B Cells, activated T Cells and B Cells, thymocytes, dendritic cells |
| CD34 | 581 IgG1 (mouse) | Fifth | Myeloid and lymphoid precursor cells |
| CD38 | LS198-4-3 IgG1 (mouse) | Fifth | Activated lymphocytes, subpopulation of B Cells, plasma cells |
| CD20 | B9E9 (HRC20) IgG2a (mouse) | Fifth | B Cells and a subpopulation of B precursor cells |
| CD19 | J3-119 IgG1 (mouse) | Fourth | Precursor and mature B Cells |
| CD45 | J.33 IgG1 (mouse) | Third | All Leukocytes |
There are more and more publications using CD200 for CLL. When reviewing publications that were using CD23 to differentiate CLL, we saw these were decreasing. In addition to that, we find that CD200 is a useful marker not only for the differentiation of CLL versus mantle cells but also in cases of atypical CLLs. With atypical CLLs, we saw that the CD23 can be dim and sometimes even negative. If we compare that to CD200, we find that CD200 is always strongly expressed in CLL, and it is either negative or very dim in mantle cells.
Again, there is CD34 in here, which is useful because, at a glance, you can see if there are myeloid or lymphoid precursor cells. If we see some abnormal immature cells, CD34 will help you identify that. There is CD38 as an activation marker and the B cell markers CD20 and CD19. We have CD45 for gating and Kappa Lambda light chains that are helpful to identify the B cell clonal population if it's present in the sample.
T cell markers
For T-cells again, there are most of the T cell markers needed. There is CD4 helper, CD2 T cell marker and NK-marker, and CD56 for the NK-cell subset. Also, CD5, a mature T cell marker, but with CLL there is aberrant expression of CD5 in those CLL cells. We have CD34 to identify any of the lymphoid and myeloid precursor cells, and immature populations, if present, can be identified.
Table 8. ClearLLab 10C System Specificity T cell Tube: Leukocyte Typing International Workshop Information for the T cell Tube Markers.
| Marker | Clone/Ig Chain (species) | Leukocyte Typing International Workshop | Reactivity |
| CD4 | 13B8.2 IgG1 (mouse) | First | Helper / inducer T Cells, monocytes, immature myeloid cells |
| CD2 | 39C1.5 IgG2a (rat) | Second | T Cells and most of the NK cells |
| CD56 | N901/NKH-1 (mouse) | Second | NK cell subset and activated T Cells |
| CD5 | BL1a IgG2a (mouse) | Third | Thymocytes, mature T Cells, subpopulation of B Cells |
| CD34 | 581 IgG1 (mouse) | Fifth | Myeloid and lymphoid precursor cells |
| CD7 | 8H8.1 IgG2a (mouse) | Second | T Cells, NK cells, subpopulation of immature myeloid cells |
| CD8 | B9.11 IgG1 (mouse) | Third | Cytotoxic / suppressor T Cells, subpopulation of NK cells |
| CD3 | UCHT1 IgG1 (mouse) | First | Mature T Cell (cytoplasmic expression in immature T Cells) |
| CD45 | J.33 IgG1 (mouse) | Third | All Leukocytes |
There is CD7, which is a T cell and NK-marker, CD8 a cytotoxic marker, CD3, which is a lineages marker, and CD45 for gating. Also included, although not a first-line marker, is TCRGamma-Delta. Gamma-Delta T cell lymphoma is very rare, but very aggressive as well. We decided to incorporate it to quickly identify these T cell lymphomas that require more aggressive therapy.
Myeloid markers
For M1 tube, the first marker is CD7, which is a T cell tube, but CD7 is aberrantly expressed in a subpopulation of immature myeloid cells, particularly in AMLs, so it's important to have that, and if you see CD7 expression, that's an aberrant expression. Then there is CD10 again, that common leukocyte antigen, and we can also see the dim staining of CD10 on the neutrophils. We have CD11B, which is on mature and immature myeloid cells, and covers granulocytes and monocytes and some NKs. There is CD13, which also covers mature and immature myeloid cells. CD14 is included, which is a strongly expressed marker on monocytes and macrophages and is weakly expressed in neutrophils. Then CD16, which is an NK cell and neutrophil and macrophage marker. Again, we have our CD34 that can help identify an immature population if it's there, CD45 for gating, plus CD64, a strong monocyte marker, all in the M1 tube.
Table 9. ClearLLab 10C System Specificity M1 Cell Tube: Leukocyte Typing International Workshop Information for the M1 Cell Tube Markers.
| Marker | Clone/Ig Chain (species) | Leukocyte Typing International Workshop | Reactivity |
| CD7 | 8H8.1 IgG2a (mouse) | Second | T Cells, NK cells, subpopulation of immature myeloid cells |
| CD10 | ALB1 IgG1 (mouse) | Third | Common acute leukemia antigen (CALLA), lymphatic precursor cells, neutrophils, subpopulation of mature B Cells |
| CD11b | Bear 1 IgG1 (mouse) | First | Mature and immature myeloid cells (granulocytes and monocytes) and NK cells |
| CD13 | Immu103.44 IgG1 (mouse) | Third | Mature and immature myeloid cells (granulocytes and monocytes) |
| CD14 | RMO52 igG2a (mouse) | First | Strongly expressed on monocytes, macrophages and weakly expressed on neutrophils |
| CD16 | 3G8 IgG1 (mouse) | Second | NK cells, monocytes, neutrophils and macrophages |
| CD34 | 581 IgG1 (mouse) | Fifth | Myeloid and lymphoid precursor cells |
| CD45 | J.33 IgG1 (mouse) | Third | All Leukocytes |
| CD64 | 22 IgG1 (mouse) | Fourth | Monocytes, macrophages |
For the M2 tube, again we're looking at the same populations of neutrophils and monocytes. There is CD15 for neutrophils, eosinophils, monocytes and macrophages, and normal myeloid precursors, and CD123, which is on hematopoietic stem cells. CD123 is widely overexpressed in various hematological malignancies including AML, B-lymphoblastic leukemia/lymphoma (BLL), and hairy cell. CD123 is expressed at both levels of the leukemic stem cells and the more differentiated leukemic blasts, and the literature states this can make for an attractive therapeutic target for AML.
In addition, there is CD117, which is a myeloid precursor cell and is also positive for mast cells. Also, CD13, which is again for mature and immature myeloid cells, and CD33 is expressed on monocytes and the myeloid precursor cells and is weak on neutrophils. Again, CD34, which is useful to identify precursor cell populations, if there is an immature population. Chances are, for the most part, the blast will be CD34, but there will be some instances where the acute leukemia will not be CD34 positive, but there are other markers to help pinpoint those. We have CD38 as an activation marker and CD19. You may ask why CD19 is in the myeloid tube but CD19 is aberrantly expressed in AMLs, particularly those with the 8;21 translocation. If you see aberrant expression of CD19 on your myeloid cells this could give you a hint that you're dealing with this specific AML with the 8;21 translocations. Plus, there is CD45 for gating.
Table 10. ClearLLab 10C System Specificity M2 Cell Tube: Leukocyte Typing International Workshop Information for the M2 Cell Tube Markers.
| Marker | Clone/Ig Chain (species) | Leukocyte Typing International Workshop | Reactivity |
| CD15 | W6D3 IgG1 (mouse) | First | Neutrophils, eosinophils, monocytes, macrophages, normal myeloid precursor |
| CD123 | 9F5 | Sixth | Hematopoietic stem cells |
| CD117 | 104D2D1 IgG1 (mouse) | Sixth | Myeloid precursor cells and masT Cells |
| CD13 | Immu103.44 IgG1 (mouse) | Third | Mature and immature myeloid cells (granulocytes and monocytes) |
| CD33 | D3HL60.251 IgG1 (mouse) | Fifth | Monocytes, myeloid precursor cells, weak on neutrophils |
| CD34 | 581 IgG1 (mouse) | Fifth | Myeloid and lymphoid precursor cells |
| CD38 | LS198-4-3 IgG1 (mouse) | Fifth | Activated lymphocytes, subpopulation of B Cells, plasma cells |
| CD19 | J3-119 IgG1 (mouse) | Fourth | Precursor and mature B Cells |
| CD45 | J.33 IgG1 (mouse) | Third | All Leukocytes |
The first IVD process controls for leukemia & lymphoma
One of the important aspects of a panel is to have internal controls to ensure the panel is performing as expected, and the way to do this is with controls. The first line of controls are internal controls. These are the normal residual cells that act as internal reference standards and can be used to determine if a marker is brighter or dimmer than expected on a specific cell population. Basically, you are comparing the brightness using your normal control; if your normal is as bright or if your abnormal is brighter, these provide clues to what these cells could be.
Again, the inclusion of the backbone and redundant markers with the panels really make it easy to track these population between the tubes. You can merge the data if you like, and you can also use it to troubleshoot any unexpected results.
CD45 is used for consistent gaining across all four tubes, and again, CD34 is consistently used across all four tubes to identify immature populations and it is possible to follow that population across all the lineages and try to determine specific lineages, whether lymphoid or myeloid.
Something that's unique to the ClearLLab 10-color system is the control cells. These are the first IVD application-matched process controls for leukemia and lymphoma. These are true process controls, which means they are processed in the same way you process patient samples. There are two phenotypes of normal phenotype, and an abnormal phenotype with lot-specific assay values that are provided for each of the ClearLLab 10-color panels. These controls provide positive reactivity for each of the 27 markers across all four tubes: The B-cell, T-cell, the M1 and the M2 tubes. Included is the Levy Jennings lot-specific templates, downloadable from the Beckman Coulter website, and these templates help monitor the performance of the controls over time within your laboratory, as well as your reagents. If there's a problem and you need to troubleshoot it, they reflect something wrong with your panel, and it's a good approach to track performance of instrument settings, panel reagents and even your technologists and their expertise at running controls.
The Levy-Jennings template assay values are used in the enhanced QC feature for Kaluza C analysis software, making it easy to analyze your patient data and control data, as well as monitor performance of the controls over time.
B cell control panel
Let’s review the data to see how these controls work with the ClearLLab individual panels, starting with the B cell control panel. This is a normal control with our B cell markers. We have CD19, CD20 and CD200. There is nice separation of Kappa Lambda, which is important to establish clonality. There is a dim CD10 that acts like a QC for our granulocytes, and there is CD5 positivity on the T-cells.
It is important to note here that these are preserved cells. They mimic normal peripheral blood, so you really have a good separation of your lymphocytes, monocytes and granulocytes; but again, this will never be perfect because the cells are preserved, but the data looks good, especially the scatter.
What's good about the abnormal is this blast-like population that you can see in purple. That is staining brightly with CD34 as most blasts do. In addition to having this abnormal population, here there are all the same normal markers associated with the B cell tube: The B cell markers, Kappa Lambda, then CD200, CD5 and CD10. These are all represented in the normal, but it's also represented in the abnormal along with an abnormal population, providing reactivity for both normal and abnormal within the tube.
T cell control panel
For our T cell panel, this is the normal control, so we don't have our blast-like population, but we have all our T cell panel markers. We have CD3, CD5, CD2, CD7, and good separation of these mutually exclusive CD4/8’s, and good staining with the Gamma-Delta. For the abnormal, there are all the same normal markers present in the T cell tubes, with the addition of that CD34 blast-like abnormal population to verify that CD34 staining.
M1 control panel
For the M1 panel for normal, we're going to switch gears and look at different populations. As this is the myeloid panel, the focus is on granulocyte and monocyte populations, and, as it's a normal, there is no CD34 staining, but there is good staining with CD16 and CD34, and dim CD10 for your granulocytes, and for monocytes you have really nice staining with CD14 and CD64 that really pulled up the monocyte population, as well as the CD14/CD11B combination. Again, there is DR on the monocytes because that becomes important in some of the differential diagnosis within the AML Leukemias especially with our APL or our AML M3.
For the abnormal on the M1 there are the same normal markers, and that CD34 positive population in here.
M2 control panel
When we look at the M2 normal, the focus is still on granulocytes and monocytes, with good staining of CD13 and CD15 on the granulocytes, and with CD33 on the monocytes, the DR and the CD38, but again no abnormal population, so no CD34 staining.
Reviewing the M2 abnormal, you see the benefit of the control, as not only are all normal populations present, but we have the granulocytes CD15 and CD13 staining, and the monocytes with DR, CD33 and CD38. You can QC the CD19 because it's positive on B-cells. In addition, there are these abnormal markers, which are CD34, CD123 and CD117, and so this blast-like population is positive for these abnormal markers.
ClearLLab Controls provide positive reactivity for all the 27 markers in the panel, both normal and abnormal, and are a good addition to any quality assurance program in the laboratory.
Compliance & Efficiency
ClearLLab 10C is the first FDA-cleared 10-color leukemia and lymphoma immunophenotyping panel. The construction of the panel is centered on developing individual tubes within lineages that can be combined to yield an informative immunophenotype. The panels are compliant with WHO guidelines and the Bethesda Conference consensus for first line markers and some second line markers.
Workflows are streamlined, as these are unitized premix panels using DURA Innovations dry reagent technology, which reduces pipetting errors because no cocktailing is required. The dry technology also delivers long stability, even up to a year, meaning labs no longer need to make cocktails every week or every month.
ClearLLab is not just a panel, it’s a comprehensive, standardized system with Navios & Navios EX flow cytometers, the panel reagents, the compensation and sample preparation reagents, quality control reagents and analysis software. Together they deliver an efficient and compliant solution for leukemia and lymphoma immunophenotyping.
References
- https://www.cancer.org/cancer/non-hodgkin-lymphoma/about/b-cell-lymphoma.html
- Wood BL et al. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry B Clin Cytom. 2007;72 Suppl 1:S14-22. https://doi.org/10.1002/cyto.b.20363
*For Non-Hodgkin’s lymphoma only