B Cells
B cells, also known as B lymphocytes, are white blood cells that mediate the production of antibodies, or immunoglobulins (Ig), in the adaptive humoral immune system. These antibodies bind to specific antigenic epitopes on invasive pathogens, marking them for elimination via phagocytic or cytotoxic mechanisms. B cells can be divided into four main categories depending on their maturation:
- Naïve – cells that have not been exposed to an antigen
- Plasma/effector – activated cells with a receptor to recognize foreign antigens and produce antibodies
- Memory – dormant, circulating cells that can initiate a faster and stronger immune response to a specific antigen that has previously been encountered
- Transitional – an intermediate stage between immature and mature B cells
B Cell Formation and Structure
B cells are approximately 8-10 µm in diameter with a large nucleus of dense heterochromatin, and a cytoplasmic border, containing mitochondria, ribosomes, and lyzosomes.1 These cells express a surface B cell antigen receptor (BCR) that binds to invasive antigens to elicit an immune response.
The maturation and B cell commitment of multipotent hematopoietic stem cells follows a progressive lineage, from common lymphoid progenitors (CLPs) to pre B cells and immature B cells, before finally becoming mature B cells.2
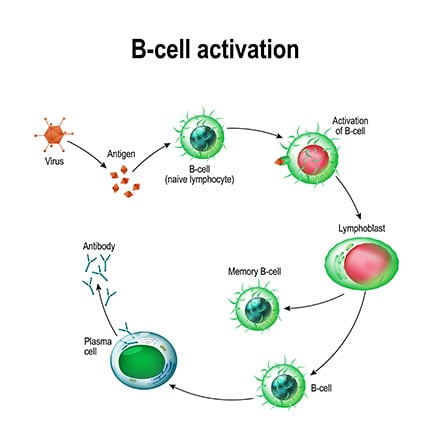
Plasma and Memory Cell Formation
Plasma cells and memory cells can differentiate from mature B cells after antigen-induced activation of a BCR. This T cell-dependent activation begins when a protein antigen binds to a BCR on the B cell surface, where the antigen is internally processed and presented by MHC-II molecules to activate T helper (TH) cells. These TH cells then secrete B cell-activating cytokines and express CD40L that interacts with CD40 receptors on the B cells, resulting in the differentiation and proliferation into plasma and memory cells.3 Plasma cells arising from this T cell-dependent activation are long-lived with a high affinity for antigens, and play a key role in maintaining serum antibody levels. In contrast, B cell activation by innate immune sensing of pathogen-associated molecular patterns (PAMPS) – such as liposaccharides and non-protein antigens from microorganisms – produces short-lived plasma cells with a low affinity for antigens, and does not initiate the formation of memory cells for a secondary response to subsequent pathogen exposures.
B Cell Function
B cells play a fundamental role in the adaptive immune system. Their primary function is antibody production, but they also act as antigen-presenting and cytokine secreting cells. B cells can be divided into discrete subsets of regulatory and effector cells based on their cytokine secretion, as regulatory cells produce immunosuppressive IL-10 and TGFβ, while effector cells can amplify immune responses with IL-2, IL-4, IL-6, or IFNγ, IL-12, and TNFα.4 In addition, B cells are regarded as antigen-presenting cells, as they take up antigens, generate MHC-II/peptide complexes, and expose them on their surface for presentation to TH cells.5
Regulatory B Cell Function
Regulatory B cells (Bregs) act as suppressors to modulate the immune system and inhibit inflammation from overexpression. To do this, they produce anti-inflammatory mediators, induce death with ligand-mediated apoptosis, and regulate the proliferation and differentiation of immune cells.6 For example, Bregs produce IL-10 to suppress the proliferation and cytokine production of T cells, and induce regulatory T cells to prevent inflammation. Individuals with multiple sclerosis have a reduced capacity to secrete IL-10, suggesting an important role for Bregs in modulating the immune response.7
Plasma Cell Function
Plasma cells secrete hundreds of thousands of antigen-specific antibodies per second into the blood and lymphatic system when activated by a pathogen. These antibodies specifically bind to the pathogen to cause inactivation, trigger the destruction of bacterial cells by complement- or NK cell-mediated lysis, and promote phagocytosis with opsonization. Recent research suggests further functions of plasma cells in the regulation of autoimmune responses, the interaction with stromal cells to initiate hematopoiesis, and gut microbial homeostasis through the production of IgA.8
Memory Cell Function
Memory cells circulate in the body in a quiescent state and do not produce antibodies until a specific antigen is re-encountered, activating pathogen-induced differentiation into plasma cells which then secrete IgG antibodies. Memory cells retain their antigens and produce a more intense antibody response to lower amounts of antigen, without any interaction of TH cells.9
The Role of B Cells in Health and Disease
Different subsets of B cells have been shown to act as important effector cells in autoimmune diseases, as they contribute to antigen presentation, regulate lymphoid tissue structure and dendritic cell function, and secrete inflammatory cytokines and chemokines, including IL-6, tumor necrosis factor-alpha (TNF-α) and lymphotoxin alpha (LT-α ).10 Loss of B cell tolerance and the dysfunctional production of antibodies is also a hallmark of autoimmune diseases, and the presence of autoantibodies in the peripheral circulation is often used as a marker for the prediction and diagnosis of these diseases.11 Increasing evidence has also shown that Bregs in particular play an important role in health by suppressing inflammation, and aberrant function can lead to many diseases including autoimmune disorders such as multiple sclerosis, allergies, cancers, and infections.12
In addition, B cells can contribute to anti-tumor immunity through the production of antibodies, the promotion of NK cells, phagocytosis by macrophages, and the priming of various T cells.13 On the other hand, they can also promote tumor development with tumor growth factors, and numerous B cell specific malignancies have been described such as non-Hodgkin lymphoma, chronic lymphocytic leukemia, B cell acute lymphocytic leukemia, and marginal cell lymphoma. B cell-depleting therapy, which targets surface molecules such as CD20 or survival factors, is often used to treat B cell cancers and autoimmune diseases.14
Emerging Research
Recent research has focused on the role of B cells in immunity and vaccine development, particularly for SARS-CoV-2. The frequency of peripheral blood B memory cells was shown to correlate with shorter COVID-19 symptom duration,15 and high immune memory persisted for up to six months in most cases.16
Tools to Study B Cells
Phenotyping of B cells - including functional studies - can be performed using fluorescence microscopy, flow cytometry and mass cytometry.17 Multiplexed isotyping panels or immunoassays can also be used to measure the profile of secreted immunoglobulin isotypes, various cytokines or chemokines that are released at different stages of B cell maturation, differentiation, and proliferation. Single cell transcriptomic analyses can be used to investigate the various subsets of B cells and their distinct developmental pathways.18
Cell Markers
Each stage of B cell maturation and activation is represented by different cell markers.CD19 is a hallmark of the B cell lineage and is expressed at all stages. CD20 is upregulated in pre B cell states but absent in pro B cells, and plasma cells are CD19+CD20-. CD10 is expressed in pro B, pre B, transitional, and germinal center B cells. In addition to CD10, cell markers CD34, CD38, and CD45 are used to immunophenotype immature cells,19 while IgM indicates mature cells prior to isotype-switch. CD27 is exclusively expressed by memory and plasma cells.20 Together with surface markers, various cytokines and chemokines secreted by B cells can be used to distinguish different subtypes, including IL-10 and TGFβ for regulatory cells, and IL-6 for memory cells.
| Cell marker | Alternative name | Type of B cell | Location |
|---|---|---|---|
| CD5 | Leu-1 | Regulatory B cell | |
| CD10 | CALLA | Pro B, pre B, transitional, germinal center B cells | Cell surface |
| CD19 | B4 | All | Cell surface |
| CD20 | MS4A1 | All (except plasma cells and pro-B cells) | Cell surface |
| CD21 | CR2, C3DR | Regulatory B cell, mature B cell | Cell surface |
| CD22 | Siglec 2 | Mature B cell | Cell surface |
| CD24 | HSA | Transitional, plasma cell, regulatory | Cell surface |
| CD25 | IL2RA | Activated | Cell surface |
| CD27 | TNFRSF7 | Transitional, Memory B cell, plasma cell | Cell surface |
| CD30 | TNFRSF8 | Activated | Cell surface |
| CD34 | My10 | Pro B | Cell surface |
| <CD38 | ADPRC1 | Expression density varies with different maturation stages and activation status | Cell surface |
| CD40 | Bp50, TNFRSF5 | Pre-B, Immature, naïve, memory B cell, plasma cell | Cell surface |
| CD45R | PTPRC, B220 | Immature, pro B, pre B | Cell surface |
| CD80 | B7-1 | Activated | Cell surface |
| CD86 | B7-2 | Activated | Cell surface |
| CD95 | Fas/APO- | all | Cell surface |
| IgM | Immature, naïve | Cell surface | |
| CD138 | SDC1 | Plasma cell | Cell surface |
| IgG | Memory B cell | Secreted, cell membrane | |
| CD319 | SLAMF7 | Plasma cell | Cell surface |
| IL-6 | Plasma cell | Secreted | |
| IgA | Memory B cell | Secreted, cell surface | |
| IgE | Memory B cell | Secreted | |
| IL-10 | Regulatory B cell | Secreted | |
| TGFβ | Regulatory B cell | Secreted | |
| IgD | Naïve, regulatory | Secreted, cell surface |
Related Products
References
1. Cano RLE., Lopera HDE. (2013). Introduction to T and B lymphocytes. In: Anaya JM, Shoenfeld Y, Rojas-Villarraga A, et al., editors. Autoimmunity: From Bench to Bedside [Internet]. Bogota (Colombia): El Rosario University Press. Chapter 5. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459471/
2. Jackson TR, Ling RE and Roy A (2021) The Origin of B-cells: Human Fetal B Cell Development and Implications for the Pathogenesis of Childhood Acute Lymphoblastic Leukemia. Frontiers in Immunology. 12:637975. doi: 10.3389/fimmu.2021.637975
3. Elgueta, R., Benson, M. J., de Vries, V. C., Wasiuk, A., Guo, Y., and Noelle, R. J. (2009). Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunological reviews, 229(1), 152–172. https://doi.org/10.1111/j.1600-065X.2009.00782.x
4. Lund F. E. (2008). Cytokine-producing B lymphocytes-key regulators of immunity. Current opinion in immunology. 20(3), 332–338. https://doi.org/10.1016/j.coi.2008.03.003
5. Adler LN, Jiang W, Bhamidipati K, Millican M, Macaubas C, Hung S-c and Mellins ED (2017) The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front. Immunol. 8:319. doi: 10.3389/fimmu.2017.00319
6. Ran, Z,. Yue-Bei, L,. Qiu-Ming, Z., and Huan, Y. (2020) Regulatory B Cells and Its Role in Central Nervous System Inflammatory Demyelinating Diseases. Frontiers in Immunology. 11:1884. doi: 10.3389/fimmu.2020.01884
7. Radomir, L., Kramer, M.P., Perpinial, M. et al. (2021). The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5. Nature Communications. 12:1893. https://doi.org/10.1038/s41467-021-22230-z
8. Pioli PD (2019) Plasma Cells, the Next Generation: Beyond Antibody Secretion. Frontiers of Immunology. 10:2768. doi: 10.3389/fimmu.2019.02768
9. Gattorno, M., and Martini, A. (2005). The Immune System and The Inflammatory Response. In Cassidy, J., Petty, R., Laxer, R., and Lindsley, C (editors). Textbook of Pediatric Rheumatology (Fifth edition). 19-63. https://doi.org/10.1016/B978-1-4160-0246-8.50009-7.
10. Musette P and Bouaziz JD (2018) B Cell Modulation Strategies in Autoimmune Diseases: New Concepts. Frontiers in Immunology. 9:622. doi: 10.3389/fimmu.2018.00622
11. Hampe C. S. (2012). B Cell in Autoimmune Diseases. Scientifica, 2012, 215308. https://doi.org/10.6064/2012/215308
12. Chekol Abebe, E., Asmamaw Dejenie, T., et al. (2021). The Role of Regulatory B Cells in Health and Diseases: A Systemic Review. Journal of inflammation research, 14, 75–84. https://doi.org/10.2147/JIR.S286426
13. Yuen, G. J., Demissie, E., & Pillai, S. (2016). B lymphocytes and cancer: a love-hate relationship. Trends in cancer, 2(12), 747–757. https://doi.org/10.1016/j.trecan.2016.10.010
14. Ramos-Casals, M., Sanz, I., Bosch, X., Stone, J. H., & Khamashta, M. A. (2012). B-cell-depleting therapy in systemic lupus erythematosus. The American journal of medicine, 125(4), 327–336. https://doi.org/10.1016/j.amjmed.2011.09.010
15. Newell KL, Clemmer DC, Cox JB, Kayode YI, Zoccoli-Rodriguez V, et al. (2021) Switched and unswitched memory B cells detected during SARS-CoV-2 convalescence correlate with limited symptom duration. PLOS ONE 16(1): e0244855. https://doi.org/10.1371/journal.pone.0244855
16. Sakharkar, M., Rappazzo, GC., Wieland-Alter, W., et al. (2021). Prolonged evolution of the human B cell response to SARS-CoV-2 infection, Science Immunology, 6, 56, /doi/10.1126/sciimmunol.abg6916
17. Boonyaratanakornkit, J., Taylor, JJ. (2019). Techniques to Study Antigen-Specific B Cell Responses. Frontiers in Immunology. 10:1694. doi: 10.3389/fimmu.2019.01694. PMID: 31396218; PMCID: PMC6667631.
18. Stewart A, Ng JC-F, Wallis G, Tsioligka V, Fraternali F and Dunn-Walters DK. (2021). Single-Cell Transcriptomic Analyses Define Distinct Peripheral B Cell Subsets and Discrete Development Pathways. Frontiers in Immunology. 12:602539. doi: 10.3389/fimmu.2021.602539
19. Bagwell, C. B., Hill, B. L., Wood, B. L., Wallace, P. K., Alrazzak, M., Kelliher, A. S., & Preffer, F. I. (2015). Human B-cell and progenitor stages as determined by probability state modeling of multidimensional cytometry data. Cytometry. Part B, Clinical cytometry, 88(4), 214–226. https://doi.org/10.1002/cyto.b.21243
20. Althuwaiqeb SA, Bordoni B. (2021). Histology, B Cell Lymphocyte. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560905/

