Biomek i-Series Automated Promega Wizard MagneSil Tfx™ Plasmid Purification System
Kirk Hetzel, Kevin Kershner, Eric Vincent, Promega Corporation, Madison, WI Joseph Lu, Bhagya Wijayawardena, Michael Hayes, Beckman Coulter Life Sciences, Indianapolis, INPlasmids are small, circular, double-stranded DNA molecules that naturally exist in bacterial cells to provide evolutionary advantages, such as antibiotic resistance. Plasmids are frequently used in molecular and synthetic biology as “vectors” to introduce novel DNA to clone, transfer, and manipulate genes within host organisms. Purification of plasmid DNA from bacterial cultures to remove proteins and other contaminants is crucial for successful downstream applications, like cloning, sequencing, transformation of bacterial cells, and transfection of mammalian cells.
The Promega Wizard MagneSil Tfx™ Plasmid Purification System is a simple, reliable method for the rapid isolation of plasmid DNA. The purified plasmid can be used in many molecular biological protocols, including Sanger sequencing and restriction enzyme digestion. Additionally, DNA purified using the Wizard MagneSil Tfx™ system contains low endotoxin levels, suitable for the transfection of mammalian cells. Purification of plasmid DNA using the MagneSil Tfx™ kit begins with alkaline lysis of bacterial cells. This is followed by clarification of the lysate and immobilization of plasmid DNA onto magnetic particles, allowing purification using a magnetic field (Figure 1). The immobilized plasmid samples are washed and then eluted with water to obtain purified plasmid DNA. As this kit employs magnetic separation rather than vacuum manifolds or centrifugation, it is well suited to automation. Here we automated the Wizard MagneSil Tfx™ System on a Biomek i-5 Multichannel liquid handler (Figure 2).
The Promega Wizard MagneSil Tfx™ System automated on a Biomek platform provides:
- Reduced hands-on time and increased throughput compared to the manual operation
- Reduction in pipetting errors compared to the manual operation
- Standardized workflow for improved results
- Quick implementation with ready-to-implement methods.
Figure 1. Workflow (A) of the Promega Wizard MagneSil Tfx™ Purification System workflow (B)
Spotlight
Biomek i5 Multichannel 96 Genomics Workstation:
System features deliver reliability and efficiency to increase user confidence and faster walk-away time compared to the manual operation:
- 300 µL or 1200 µL Multichannel head with 1-300µL and 1-1200 µL pipetting capability
- Enhanced Selective Tip pipetting to transfer custom array of samples
- Independent 360˚ rotating gripper with offset fingers
- High deck capacity provided by 25 positions and separate locations for trash
- Orbital Shakers, heating/cooling Peltiers, and 96 channel tip washing for sample processing control.
Figure 2. Biomek i5 Multichannel Automated Liquid Handler
Automated method
After initial deck setup, the automated Wizard MagneSil Tfx™ method processes 96 samples in less than 2 hr according to Biomek 5 software time estimation (Table 1). The 96 channel multichannel head of Biomek i5 enables efficient processing of 96 samples. In situations where multiple plates are processed, the method can be automated on the Biomek i7 workstation. The Demonstrated Method Interface consists of Biomek Method Launcher (BML), Method Option Selector (MOS), and Guided Labware Setup (GLS), simplifying the method editing, modifications, and setup (Figures 3-5).
| PROCESS | TIME (96 SAMPLES; I5 MC) |
| Instrument setup | 30 min |
| Method run | 1 hr 40 mins |
| Total | 2 hrs 10 mins |
Table 1. Run times for Promega Wizard MagneSil Tfx™ Plasmid Purification System automated on Biomek i5 Multichannel workstation. Timing does not include reagent thawing and homogenization. Automated method runtime is calculated by Biomek 5 software.
1. Biomek Method Launcher (BML):
BML is a secure interface for executing methods without affecting method integrity. It allows the users to remotely monitor the progress of the run. The manual control options provide the opportunity to interfere, if needed.
Figure 3. Biomek Method Launcher provides an easy interface to start the method.
2. Method Options Selector (MOS)
MOS enables selection of plate processing and sample number options to maximize flexibility, adaptability and the ease of method execution. To reduce the time of manual setup, the method provides options to aliquot reagents into processing plates, from the reservoirs.
Figure 4. Biomek Method Options Selector indicates sample number and processing options.
3. Guided Labware Setup (GLS)
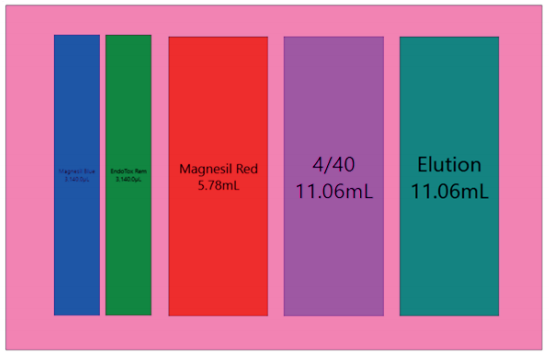
GLS is generated based on options selected in the MOS, and provides the user specific text and graphical setup instructions with reagent volume calculation and step by step instructions to prepare reagents.
Figure 5. Guided Labware Setup indicates reagent volumes and guides the user for correct deck setup.
Methods
E. coli cells were cultured overnight at 37°C in LB medium. Once an OD600 of 2.0 was achieved, cells were transferred to 16 individual wells of a 96-well deep well plate at 1 mL per well. Cells were pelleted by centrifugation for 15 minutes at 1,500 × g. The resulting supernatant was discarded, and the collected cells were used immediately in the automated plasmid purification method (Figure 1).
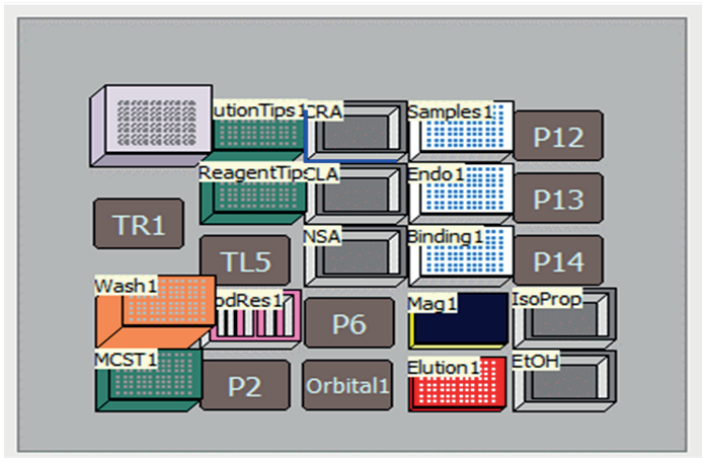
Each of the following liquid handling steps was performed using a Biomek i5 Multichannel liquid handler (Figure 2). For each well, cell pellets were resuspended in 90 µL of Cell Resuspension solution. Lysis was performed by adding 120 µL of Cell Lysis Solution, followed by the addition of 120 µL of Neutralization Solution. Following lysis, 25 µL per well of MagneSil BLUE was added, well contents were transferred to a new 96 well plate, and plate was placed on a MagnaBot® FLEX 96 Magnetic Separation Device. The resulting clarified lysate was added to a new 96 well plate containing 25 µL of Endotoxin Removal Resin, mixed by orbital shaker, and returned to the MagnaBot. The resulting supernatant was transferred to a 96-well plate containing 50 µL of MagneSil RED and 350 µL of 100% isopropanol, mixed by orbital shaker for 5 minutes and returned to the MagnaBot, and the supernatant was discarded. The MagneSil RED resin was washed with 100 µL of 4/40 Wash Solution followed by three washes with 100 µL of 80% ethanol. The supernatant was discarded after each wash step. The MagneSil resin plate was then dried to remove excess ethanol for 7.5 min using a heating ALP. After drying, DNA was eluted by addition 100 µL of Nuclease-Free Water, and the MagnaBot was used to separate eluted DNA supernatant from resin.
After Biomek i5 automated purification using the Wizard MagneSil TFX system, DNA quality and quantity was assessed using a NanoDrop 2000TM (Thermo-Fisher Scientific).
Figure 6. Biomek i5 deck layout of the automated Wizard MagneSil Tfx™ plasmid purification method
Results
In contrast to manual protocols, once the instrument was set up and the run was initiated, no user interventions were required for the purification process, as compared to ~1.5 hr of manipulation on the bench, according to manufacturer’s instructions (3). To estimate nucleic acid purity, NanoDrop calculates the ratio of the absorbance by the nucleic acid to the absorbance of the contaminants. The automated protocol yielded plasmids of good A260/ A280 ratios (1.88–1.92; Table 1) indicating high purity of the plasmid DNA. The concentration of the purified plasmids ranged from 146.4 ng/µL to 199.2 ng/µL (Table 2). The low variability in between well DNA concentration and quality indicates consistent pipetting of Biomek (%CV: Concentration: 9%, Quality 0.7%).
| Sample | Concentration (ng/µL) | 260/280 |
| 1 | 184.8 | 1.88 |
| 2 | 199.2 | 1.92 |
| 3 | 165.1 | 1.9 |
| 4 | 177.0 | 1.89 |
| 5 | 144.3 | 1.91 |
| 6 | 161.6 | 1.9 |
| 7 | 152.5 | 1.89 |
| 8 | 154.6 | 1.9 |
| 9 | 174.3 | 1.91 |
| 10 | 169.0 | 1.9 |
| 11 | 163.2 | 1.9 |
| 12 | 182.1 | 1.92 |
| 13 | 151.0 | 1.93 |
| 14 | 167.9 | 1.91 |
| 15 | 148.6 | 1.89 |
| 16 | 146.4 | 1.92 |
Table 2. DNA quantity and quality determined by NanoDrop 2000TM (Thermo Fisher Scientific).
Summary
High quality DNA is required for many downstream applications, and plasmid purification continues to be a crucial process in modern molecular biology and drug development laboratories. As an increasing number of therapies are utilizing biologics and engineered antibodies, there is more demand for high-throughput plasmid and clone processing. Automation of plasmid purification can improve throughput and provide consistent highquality results, as compared to manual processing. Here we demonstrate the automation of the Promega Wizard MagneSil Tfx™ System using a Biomek i-5 Multichannel Automated Liquid Handler in 96-Well format in under 2 hours. Following automated purification, the resulting DNA was low in common impurities, such as RNA, protein, and endotoxins, making it suitable for transfection or other downstream applications. Automation enabled efficient extraction with no user time required after loading the deck. The Biomek Method Launcher provides a user-friendly interface to run the method, thus decreasing setup errors, while providing quick implementation, reducing the time required to execute the automation.
References
- Wils, P., Escriou, V., Warnery, A., Lacroix, F., Lagneaux, D., Ollivier, M., Crouzet, J., Mayaux, J.F. & Scherman, D. (1997). Efficient purification of plasmid DNA for gene transfer using triple-helix affinity chromatography. Gene Therapy: 4, 323–330
- Stadler, J., Lemmens, R. & Nyhammar, T. (2004). Plasmid DNA purification. The Journal of Gene Medicine. https://doi.org/10.1002/jgm.512
- Technical Bulletin: Wizard MagneSil Tfx™ System https://www.promega.com/ products/nucleic-acid-extraction/plasmid-purification/wizard-magnesil-tfxsystem/?catNum=A2380#protocols