ClearLLab 10C Panel Markers and how they are combined
Mike Keeney London Health Sciences Centre, Victoria Hospital, London, ON, Canada, and Sandra Hernandez Beckman Coulter Life Sciences, Miami, FL 33196
Introduction
Flow cytometric immunophenotyping remains an indispensable tool for the diagnosis, classification, staging, and monitoring of hematologic neoplasms. There have been significant advances in flow cytometry instrumentation and availability of an expanded range of antibodies and fluorochromes that have improved the ability to identify different normal cell populations and recognize phenotypic aberrancies.
ClearLLab 10C* Panels consist of a set of four 10-color reagents in dry unitized format, composed of antibodies directed against T, B, NK and Myeloid lineage antigens. These panels are designed to include the primary antibodies which are lineage oriented as per the 2006 Bethesda consensus. The combinations are intended to identify the leukocyte subpopulations that express these antigens alone or in specific co-expression patterns, and are designed to work with both normal samples and samples coming from any patient having or suspected of having the following hematopoietic neoplasms: chronic leukemia, acute leukemia, non-Hodgkin's lymphoma, myeloma, myelodysplastic syndrome (MDS), and/or myeloproliferative neoplasms (MPN). The specific choices and combinations in the ClearLLab 10C* Panels are based on the guiding principles of1 addressing the clinical indications,2 accounting for all major cell populations present in the specimen, and3 providing sufficiently comprehensive identification of all major categories of hematopoietic cell populations in both normal and neoplastic states.1-10 The normal cells within the sample act as an internal reference and can be used to determine whether a marker is brighter or dimmer than expected on a specific cell population. The inclusion of a backbone and redundant markers within the panels makes it easier to track populations between tubes, merge the data and troubleshoot unexpected results. In the panel the backbone of CD45 and CD34 are included in each tube. CD45 is used for consistent gating across all the four tubes. CD34 in combination with weak CD45 positivity can be used to confirm the hematopoietic origin of cells. Other redundant markers have been included in the panels such as CD13 and HLA-DR, in both M1 and M2 tubes. CD10 and CD19 are included in the lymphoid and myeloid tubes. CD19 in the myeloid tube is especially useful as it is aberrantly expressed in AML associated with t(8:21).2 CD7 is included in the T cell tube as well as in the M1 cell tube to detect aberrant expression of CD7 in AML. CD200 can be useful in the differential diagnosis of B-cell neoplasms in particular atypical CLL vs Mantle Cell Lymphoma and was included in the B cell tube instead of CD23.11
Both mature and immature markers are combined in the panels to provide efficiency as well as allowing for the identification of all cells in the tubes. The construction of the panel is centered on developing individual tubes within lineages that can be combined to yield an informative immunophenotype.
1Pacific Blue. 2APC-Alexa Fluor 700. 3APC-Alexa Fluor 750.
The following case study obtained from clinical trial data highlights the utility of the ClearLLab 10C* panel.
Case Study
An 85-year-old male with known chronic lymphocytic leukemia for the past 10 years presents to a follow up appointment with anemia and bone pain. A complete blood count (CBC) and bone marrow aspirate and biopsy are collected for morphology. Additionally, a bone marrow aspirate sample is submitted for flow cytometric immunophenotyping using ClearLLab 10C* Panels.
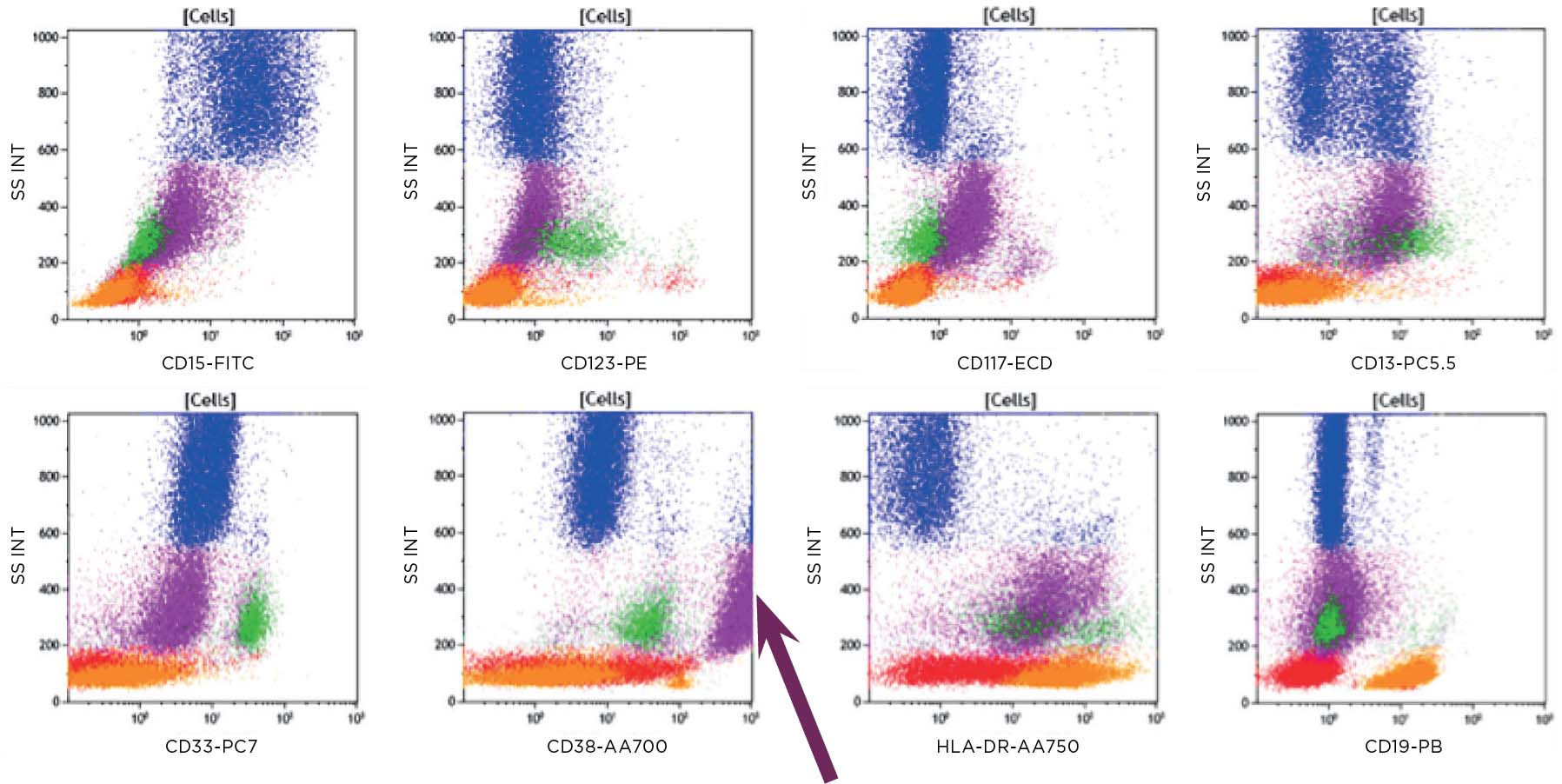
Flow cytometry was performed, and results were available within 2 hours, before the aspirate and biopsy results were available. Results for key markers are shown below.
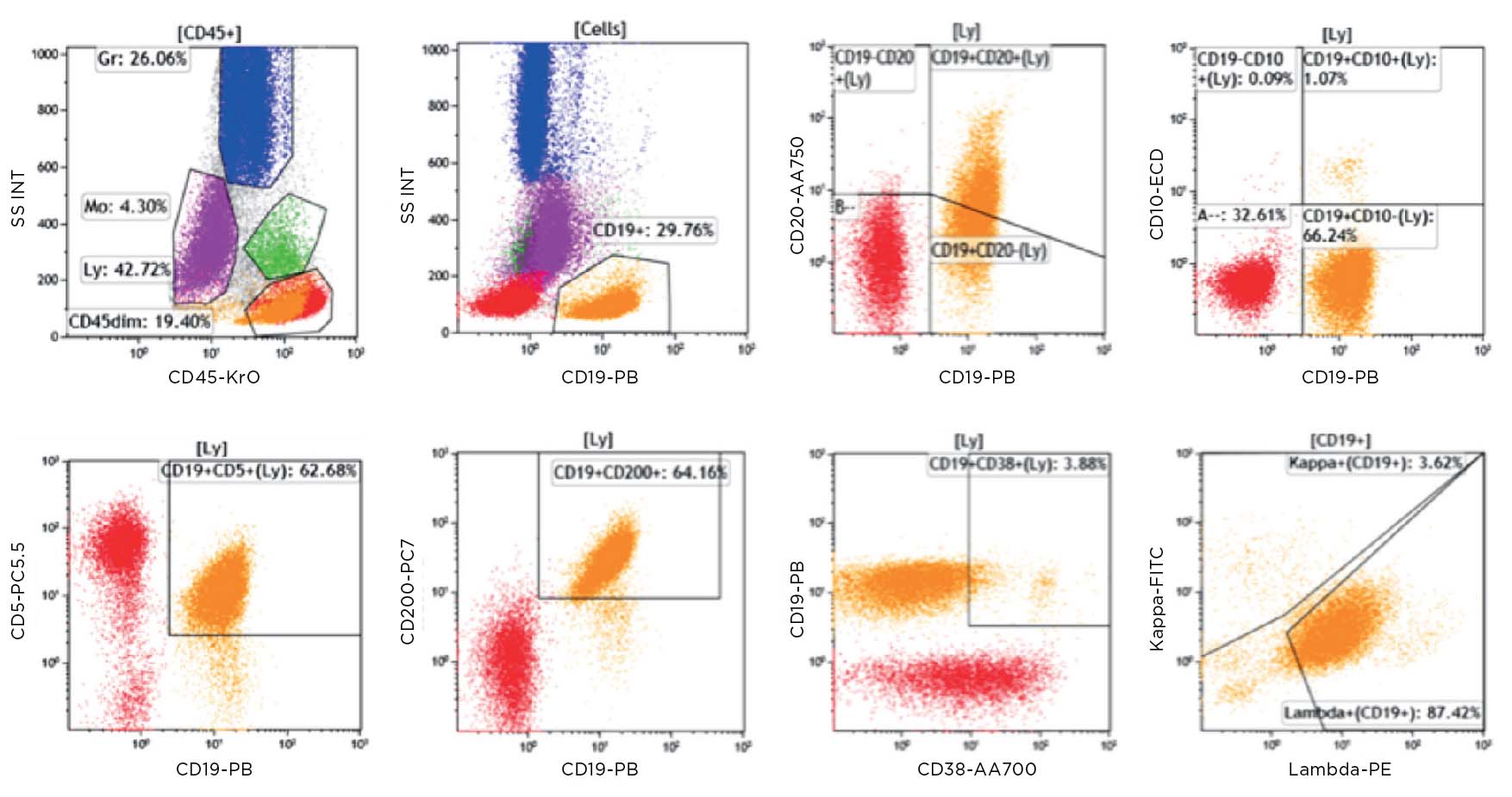
Figure 1a: Key: Lymphocytes red, B cells orange, Monocytes green, Granulocytes blue, CD45 dim purple.
Population of CD19+ cells (orange) dim/negative CD20, CD10neg, CD5 pos/dim, CD200 bright, CD38neg, lambda+. Consistent with a chronic lymphocytic leukemia population in this known CLL.
Figure 1b: The CD45 dim population (purple) is dim/negative, CD20, negative for CD19, CD5 and CD10. The bright CD38 positivity causes the CD34 to appear positive. However the CD45 dim population in this case appeared to react with the APC in each tube. Light chain expression is not interpretable on the CD45 dim population.
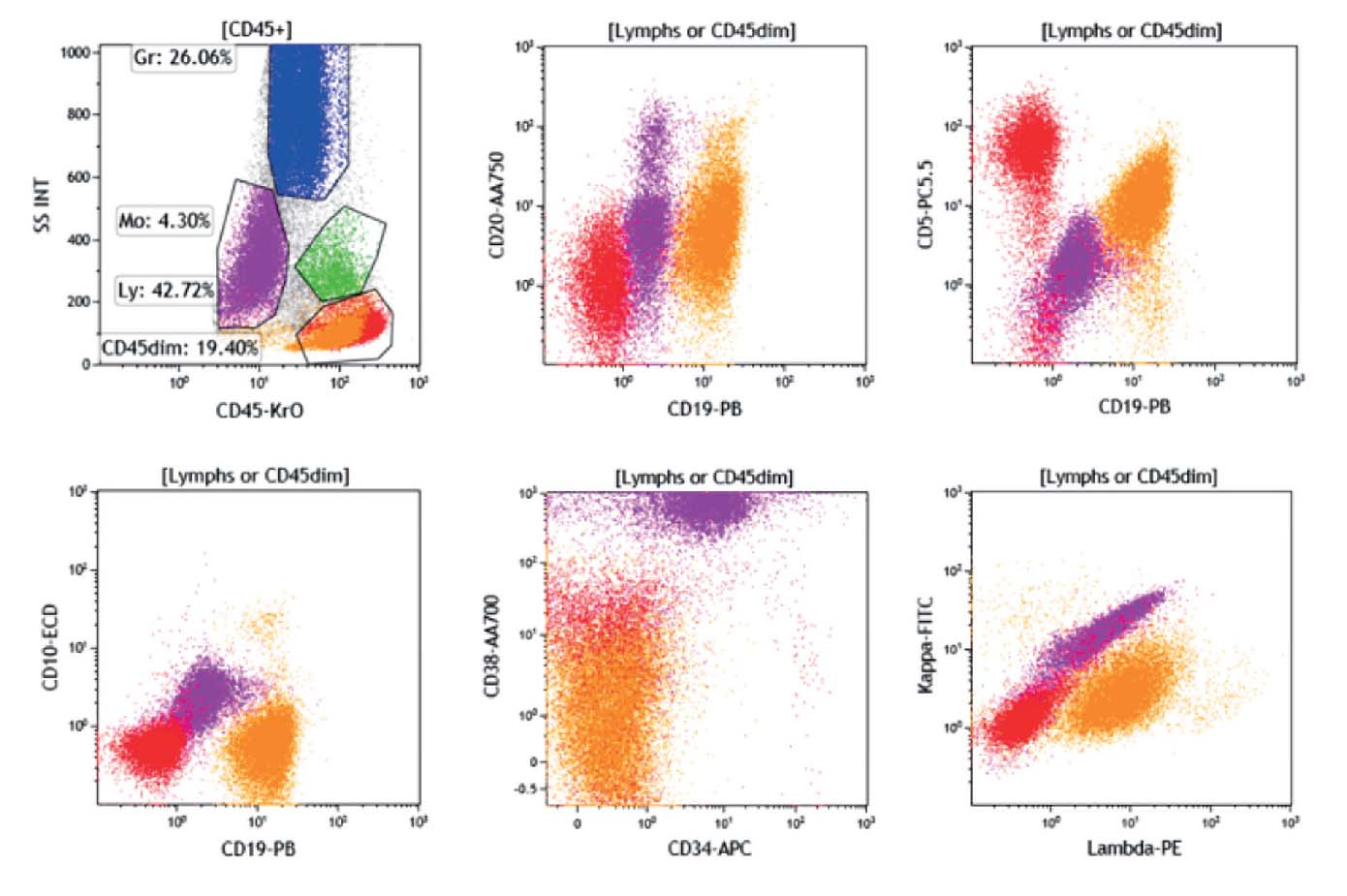
Figure 2: The B cells (orange) are CD5 dim as seen in the B tube. The CD45 dim population (purple) is negative for all T cell markers except CD56.
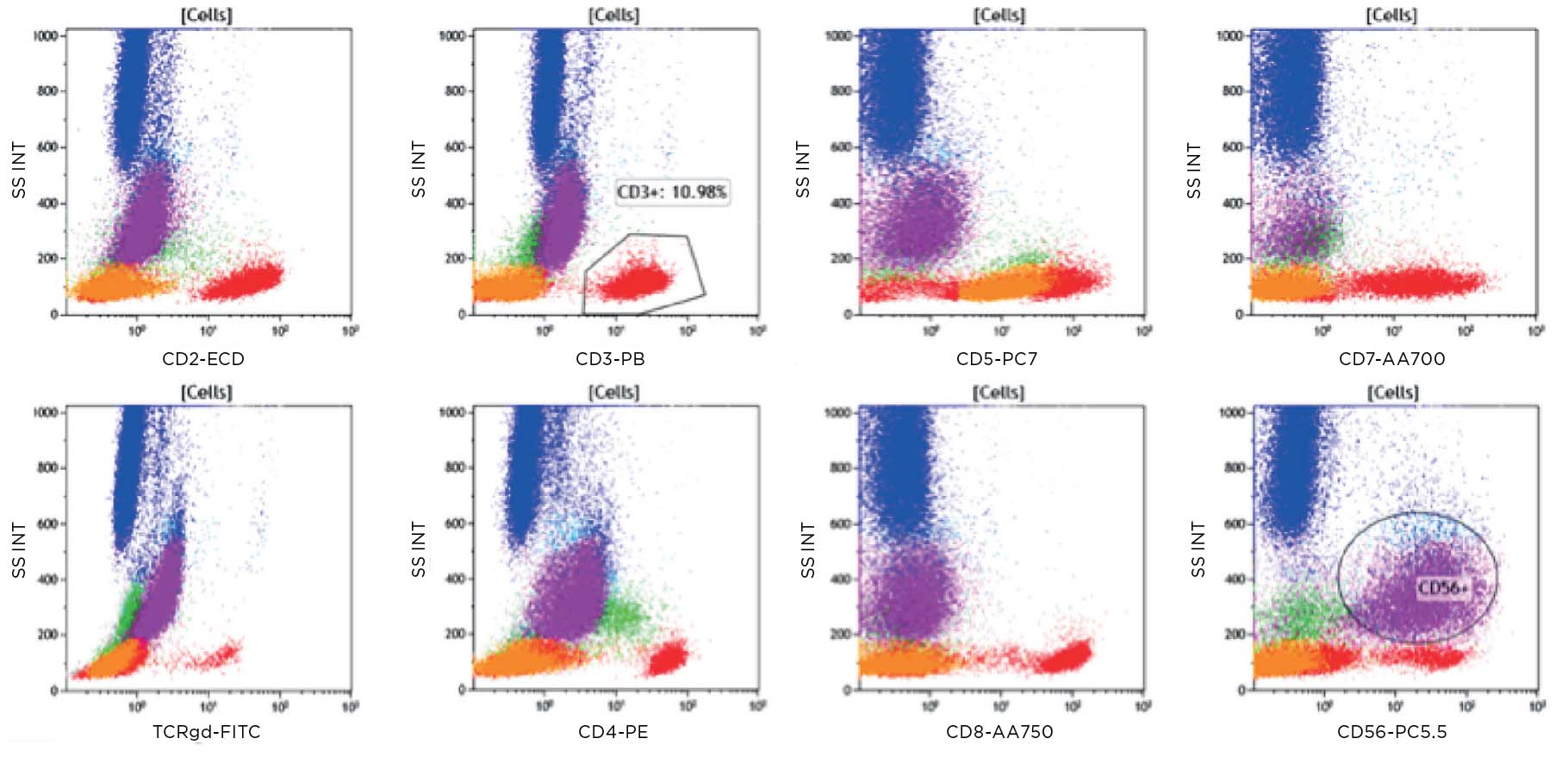
Figure 3: The CD45 dim population (purple) is positive for CD13 and HLA-DR.
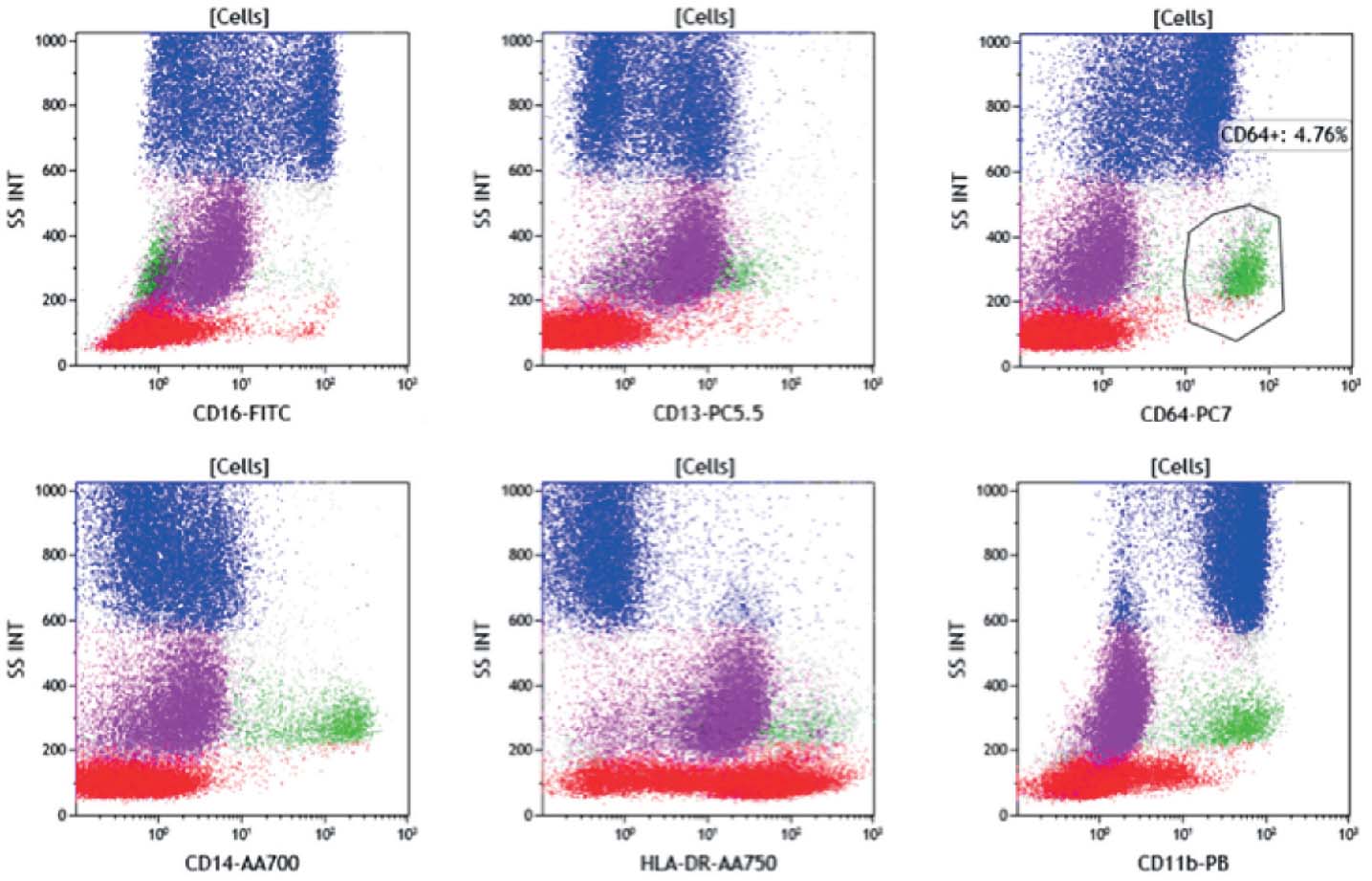
Figure 4: The CD45 dim population (purple) appears to be weakly CD117 positive, CD13 positive and bright CD38 (arrow).
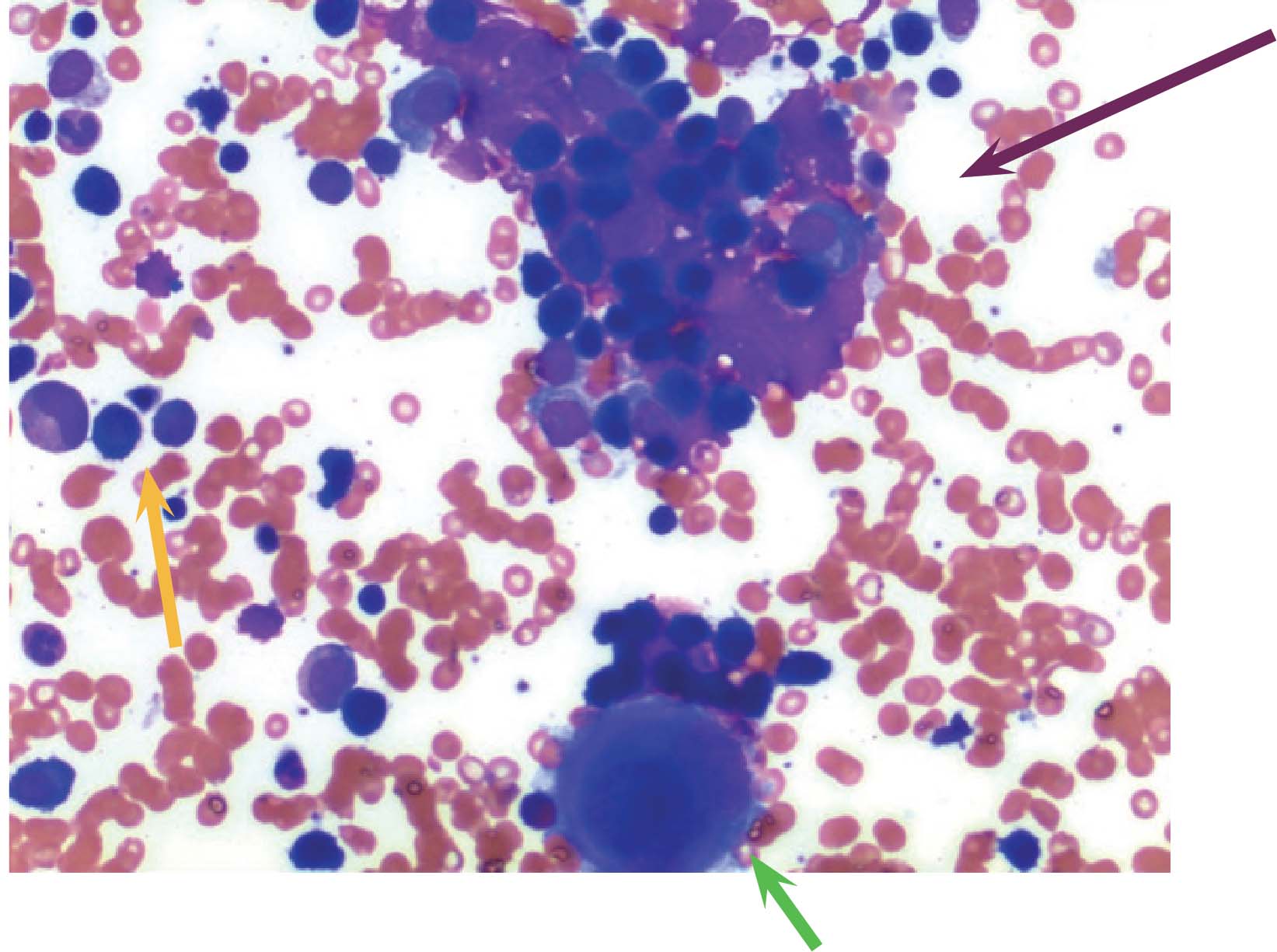
Figure 5: H&E stain of bone marrow aspirate showing mainly small lymphocytes (orange arrow) and clumps of possible plasma cells (purple arrow). Cell at bottom center is a megakaryocyte (green arrow).
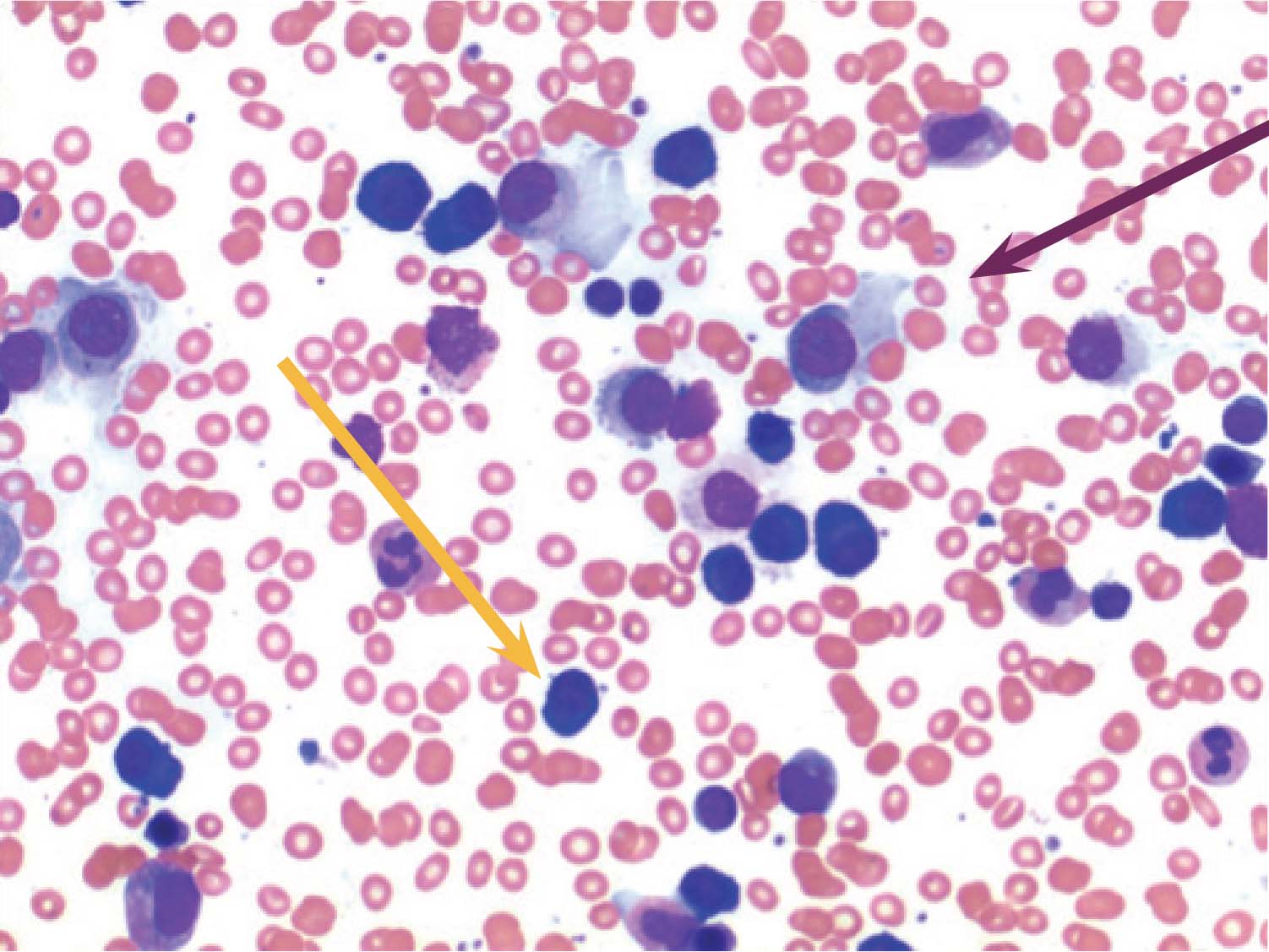
Figure 6: H&E stain of bone marrow aspirate High power field (x100). Small lymphocytes (orange arrow) and plasma cells (purple arrow) are clearly seen.
Discussion
This 85-year-old male who attended a routine follow up appointment for his long-standing low-grade chronic lymphocytic leukemia, which had been untreated, complained of bone pain and reported that he had been suffering from night sweats and weight loss over the past month. Flow cytometry showed the expected CLL population CD19+, CD5+, CD20 dim, with dim monoclonal lambda expression. The CD45 dim population was positive for CD38+++, HLA-DR, CD56, CD13, and dim/negative CD20. Other B, T and myeloid markers were negative and light chain expression was not interpretable; however follow up studies showed these to be positive for kappa on cytoplasmic staining. Taken together with morphology, with >20% plasma cells noted in the bone marrow, this case represents CLL that has developed a separate clone leading to myeloma in the same patient.
Note: the CLL population expressed lambda light chains whereas the plasma cell population expressed kappa light chains, confirming two separate disease states and not evolution of the low-grade CLL to myeloma.
Discover our comprehensive casebook
Conclusion
Flow cytometric immunophenotyping can provide a sensitive tool to aid in the identification of the presence of hematologic malignancy and assist in demonstrating the absence of disease. The ClearLLab 10C* Panels are intended for in vitro diagnostic use for qualitative identification of various cell populations by multiparameter immunophenotyping. These reagents are used as an aid in the differential diagnosis of hematologically abnormal patients having or suspected of having the following hematopoietic neoplasms: chronic leukemia, acute leukemia, non-Hodgkin's lymphoma, myeloma, myelodysplastic syndrome (MDS), and/or myeloproliferative neoplasms (MPN). Interpretation of the results should be confirmed by a pathologist or equivalent professional in conjunction with other clinical and laboratory findings.
References
- 2006 Bethesda International Consensus Recommendations on the Immunophenotypic Analysis of Hematolymphoid Neoplasia by Flow Cytometry, Cytometry 2007 72B:S14-22.
- WHO 2008 Classification of Tumours of Hæmatopoietic and Lymphoid Tissues, 4th Edition, 2008, IARC Press.
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Swerdlow SH, et al. Blood. 2016;127:2375-90.
- The 2016 revision of the World Health Organization classification of myeloid neoplasms and acute leukemia. Arber DA, et al. Blood 2016 127:2391-2405.
- Braylan RC, Orfao A, Borowitz MJ and Davis BH. 2001. Optimal number of reagents required to evaluate hematolymphoid neoplasias: Results of an international consensus meeting. Cytometry, 46:23-27.
- 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: recommendations for training and education to perform clinical flow cytometry. Greig B, Oldaker T, Warzynski M, Wood B. Cytometry B Clin Cytom. 2007;72 Suppl 1:S23-33.
- 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Wood BL, Arroz M, Barnett D, DiGiuseppe J, Greig B, Kussick SJ, Oldaker T, Shenkin M, Stone E, Wallace P. Cytometry B Clin Cytom. 2007;72 Suppl 1:S14-22.
- 2006 Bethesda International Consensus recommendations on the flow cytometric immunophenotypic analysis of hematolymphoid neoplasia: medical indications. Davis BH, Holden JT, Bene MC, Borowitz MJ, Braylan RC, Cornfield D, Gorczyca W, Lee R, Maiese R, Orfao A, Wells D, Wood BL, Stetler-Stevenson M. Cytometry B Clin Cytom. 2007;72 Suppl 1:S5-13.
- 2006 Bethesda International Consensus Conference on Flow Cytometric Immunophenotyping of Hematolymphoid Neoplasia. Stetler-Stevenson M, Davis B, Wood B, Braylan R. Cytometry B Clin Cytom. 2007;72 Suppl 1:S3.
- Flow Cytometric Immunophenotyping for Hematologic Neoplasms. F.E. Craig, K.A. Foon. Blood. 2008; 111; 3941- 3967.
- Diagnostic Usefulness and Prognostic Impact of CD200 Expression in Lymphoid Malignancies and Plasma Cell Myeloma, Alapat D, Coviello-Malle J, Owens R, Qu P, Barlogie B, Shaughnessy JD, Lorsbach RB, American Journal of Clinical Pathology, Volume 137, Issue 1, 1 January 2012; 93-100.
*CE-IVD For In Vitro Diagnostic Use. For Non-Hodgkin’s lymphoma only.