21 CFR part 11 Data Integrity for On-line WFI Instruments
Abstract
A recent report indicates that circa 79% of 483 warning letters issued by the FDA to the pharmaceutical industry in 2016 cited deficiencies in data integrity1 . The FDA outlines their expectations for quality critical instrumentation in the GMP environment in their 21 CFR Part 11 ruling2. This paper takes a look at how quality-critical on-line Total Organic Carbon (TOC) and conductivity instrumentation for WFI (water for injection) quality control can be configured to help companies comply with the FDA’s expectations on data integrity, including the use of Microsoft Active Directory and PDF data export.
Background
TOC and conductivity are two of the four key quality attributes defined for WFI and PW (purified water) in the United States Pharmacopoeia3. On-line analyzers such as the ANATEL PAT700 from Beckman Coulter can be validated to comply fully for both of these key quality attributes according to the pharmacopoeial requirements.
The FDA Process Analytical Technologies (PAT) initiative4 encouraged the pharmaceutical industry to invest in process control instrumentation to ensure in-process quality control rather than relying so heavily on final product quality testing. Right first time is encouraged as final product quality testing cannot be applied 100% because the tests are typically destructive. To this aim, many pharmaceutical manufacturers are connecting their on-line TOC analyzers to their factory control systems so that any potential TOC or conductivity excursions detected can be used to halt production and prevent the chance of potentially contaminated water being mixed with valuable active pharmaceutical ingredients.
With the revision to the European Pharmacopoeia (EP) chapter on WFI now permitting the production of WFI from double pass reverse osmosis (RO) and ultra-filtration5, TOC and conductivity monitoring of pharmaceutical water systems takes on an increasing significance due to the perceived risk of potential contamination break-through in the RO system compared to the secure barrier afforded by a water still.
FDA ALCOA guidance
In their 2003 guidance on the implementation of their 21 CFR part 11 data integrity rule, the FDA use the acronym ALCOA, where they define good data integrity practice as creating records that are attributable to the technician carrying out the testing, are legible, are created contemporaneously, original and accurate.

The FDA 21CFR part 11 ALCOA definition of complete, consistent and accurate data
Attributable can be interpreted to mean that records should include an electronic ‘signature’ to link them to the instrument/person that made the measurement and they should also include a reference to the water system being tested and the date and time it was taken. This implies electronic signatures for users signed on to the system. Control over electronic signature format can be site specific and is usually controlled by the IT department using Microsoft Active Directory controls. Ideally the on-line instrumentation should be configured to follow Active Directory controls to ensure correct electronic signature format according to the site-specific rules.
FDA 21 CFR part 11 : ALCOA
Legible records
The record of course is required to be legible, which implies that hand-written records are not acceptable. The FDA goes on to suggest that electronic records should be stored in a format that is open and can be read on many computing formats so that it will be accessible and readable for years to come. The FDA recommends typical formats such as PDF, XML or SGML.
Contemporaneous
The word contemporaneously implies that the electronic records should be created immediately the sample is measured, implying that manual transcription of paper records is not good practice and that collating paper records and then manually transcribing them into electronic format at a later time or date is not good practice either.
Naturally there is a danger with every transcription of test results from one form to another. Even scanning multiple paper records into electronic format runs the risk of duplication or missed scans. So the FDA recommends that the electronic record should be the original record created when the test was completed. Obviously, manually transcribed records are the riskiest, attracting the biggest opportunity for human error.
Finally the A in ALCOA. Naturally the electronic records should be accurate. This implies that the process for capturing those electronic records should be robust, i.e. manual calculations and manual data entry where opportunities for human error exist should be avoided.
Attributable records
Attributable is the ‘A’ in ALCOA. Electronic records generated by on-line instruments should contain information that links the data to the instrument used to make the measurement and the time and date of the measurement.
On-line instruments such as the ANATEL PAT700 that are capable of also analyzing grab samples should have the data generated by the grab sample analysis allocated to the user who carried out the test using electronic signatures.
There should be multi-level users with day-to-day users not needed to log on to view current on-line TOC results, but users looking to make changes to settings or carry out calibrations or system suitability tests should be required to log on.
Users logged on to the system should be automatically logged off after a configurable time period of inactivity.
Users should be forced to change their passwords in a pre-determined, regular timeframe and should not be allowed to re-use previous passwords they may have already used.
Ideally, the controls imposed on users by the site IT team and defined in the Microsoft Active Directory control should be implemented in the on-line TOC analyzer too.
Data repositories
In their guidance, the FDA are keen to emphasise that the 21 CFR part 11 ruling applies only to the data historian where electronic records are kept. The danger with on-line instruments with their own local data historian built in is that they could attract the full requirements of the 21 CFR part 11 ruling. Analyzers such as the PAT700 avoid this issue by allowing the local data historian to be disabled, thus ensuring that it does not attract the full 21 CFR part 11 requirement as a data archive for electronic records.
Traditionally, data from on-line TOC analyzers was stored in validated Supervisory Control And Data Acquisition (SCADA) or Distributed Control Systems (DCS) systems, making process control improvements challenging and adding a further significant amount of change control. More modern approaches keep quality critical data records in a separate secure archive, leaving SCADA and DCS systems dedicated to process control only and more agile. In support of this the PAT700 can be configured to automatically send PDF electronic records via secure FTP over Ethernet, thus satisfying the ALCOA requirements for electronic records to be legible, contemporaneous, original and attributable.
Data archiving 0f reports to a remote secure data archive over Ethernet via secure FTP can be automated, with PDF files exported automatically at pre-determined intervals.
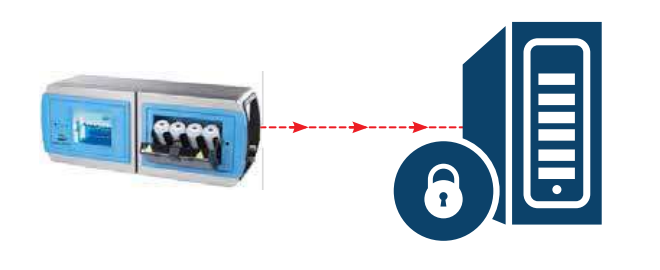
Fig. 3 Beckman Coulter ANATEL PAT700 exports PDF file straight to data archive via Ethernet
Manual SOPs vs electronic SOPs
In the ANATEL PAT700 the ‘A’ for accuracy in ALCOA is satisfied as manual processes are eliminated with all necessary SOPs automated and pre-programmed into the analyzer in electronic SOP format. There are no manual calculations when performing calibrations or system suitability testing and no manual data entry as certified standards values, lot numbers and expiry dates are automatically transferred into the analyzer from RFID tags located on the standards bottles themselves.
Manual calculations
Human error when performing manual calculations for pass/fail reports can impact the ‘A’ for accuracy and ‘C’ for contemporaneous in ALCOA. Ideally, the calculations for pass/fail should be built into the analyzer so that automatic pass/fail reports can be calculated and generated from the analyzer itself. The PAT700 contains the pass/fail criteria and automatically generates pass/fail reports in PDF format directly, fulfilling the requirement in ALCOA for accurate, contemporaneously generated records.
Much attention is paid to the security of the final electronic record, but there are many opportunities to generate incorrect records in a manual process including manual data entry and manual calculations.
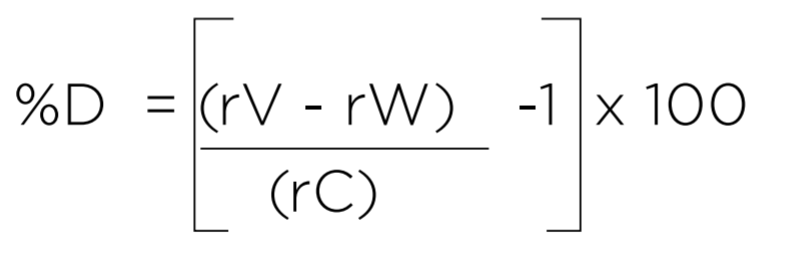
Where:
rV = Average TOC response for three measurements of the Sucrose Validation Standard
rW = Average TOC response for three measurements of the Sucrose Validation Standard
rC = Certified TOC value from the Certificate of Analysis for the Validations Standard
Calibrations
The same can be said for any calibrations carried out using manual SOPs and manual calculations. The opportunity for human error is high. The Beckman Coulter ANATEL PAT700 contains all necessary SOPs in electronic format and calibration standards automatically import their certified value, their lot number and their expiry date into the PAT700 via RFID and this data appears in the calibration report in pdf format.

Re-training vs. robust processes
Following data errors, a typical response is to mandate re-training for the team. However, the industry and the FDA is gradually coming to the conclusion that this does not solve the problem it merely treats the symptoms for a short while until human error starts to creep in again and that the correct way forward is to reduce manual steps in the SOP in order to reduce the human errors and make the whole process more robust.
Conclusion
Critical on-line water quality instrumentation is becoming even more important as the rules on WFI generation are relaxed in the European Pharmacopoeia. Manual calculations and paper-based SOPs allow human error to creep in. Re-training is just treating the symptoms, not curing the problem. The technology for making these instruments more robust by automating SOPs and eliminating manual calibrations exists in instrumentation optimized for pharmaceutical quality control such as the Beckman Coulter ANATEL PAT700 TOC and conductivity analyzer. With a focus on cost control and optimization, users would do well to consider making their on-line quality control instrumentation more robust to prevent loss of valuable active pharmaceutical ingredient product, particularly those in the biopharmaceutical industry.
References
- Pharmaceutical Online, An Analysis Of FDA FY2016 Drug GMP Warning Letters By Barbara Unger, Unger Consulting Inc. https://www.pharmaceuticalonline.com/doc/an-analysis-of-fda-fy-drug-gmp-warning-letters-0001
- U.S. Department of Health and Human Services Food and Drug Administration Guidance for Industry, Part 11, Electronic Records; Electronic Signatures — Scope and Application August 2003 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- U.S. Department of Health and Human Services Food and Drug Administration United States Pharmacopoeia U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA
- U.S. Department of Health and Human Services Food and Drug Administration Guidance for Industry PAT — A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance September 2004 U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Office of Regulatory affairs (ORA) Division of Drug Information, HFD-240 Center for Drug Evaluation and Research Food and Drug Administration 5600 Fishers Lane, Rockville, MD 20857 USA https://www.fda.gov/downloads/drugs/ guidancecomplianceregulatoryinformation/guidances/ucm070305.pdf
- Council of Europe European Directorate for the Quality of Medicines & Healthcare European Pharmacopoeia (Ph. Eur) 9th Edition. EDQM Council of Europe, 7 allée Kastner, CS 30026, F-67081 Strasbourg, France https://www.edqm.eu/en/european-pharmacopoeia-9th-edition
Author Biography
Tony Harrison is a Compliance and Applications Specialist for Beckman Coulter Life Sciences. Tony has spent the last twelve years in applied metrology in the pharmaceutical and healthcare manufacturing industries. Prior to that, he worked for companies providing process control automation solutions for manufacturing industries.
Tony was joint-editor of the ISPE Guide to Ozone Sanitization of Pharmaceutical Water Systems and was also chief editor of the PHSS Best Practice Guide for Cleanroom Monitoring.
Tony is a well-known international speaker and has provided educational seminars on pharmaceutical water quality, parenteral particle testing, ozone sanitization for water systems and cleanroom monitoring in UK, France, Italy, India, Germany, Malaysia, China, USA, Scandinavia, Ireland, Hungary, Switzerland, Indonesia, Belgium, Greece, Switzerland, Turkey, Egypt and Denmark.

